Early stage prediction of bone regeneration using FEA and cell differentiation algorithms with 3D-printed PLA and PCL scaffolds: modeling and application to dorsal double-plating in distal radius fractures
- PMID: 40536605
- PMCID: PMC12177956
- DOI: 10.1186/s41205-025-00278-7
Early stage prediction of bone regeneration using FEA and cell differentiation algorithms with 3D-printed PLA and PCL scaffolds: modeling and application to dorsal double-plating in distal radius fractures
Abstract
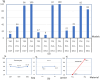
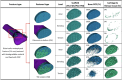
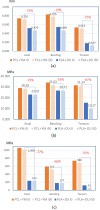
This study introduces an advanced framework that integrates biphasic cell differentiation bone remodeling theory with finite element (FE) analysis and multi-remodeling simulation to evaluate the performance of 3D-printed biodegradable scaffolds for bone defect repair. The program incorporates a time-dependent cell differentiation stimulus (S), accounting for fluid-phase shear stress and solid-phase shear strain, to dynamically predict bone cell behavior. The study focuses on polylactic acid (PLA) and polycaprolactone (PCL) scaffolds with diamond (DU) and random (YM) lattice designs, applied to a dorsal double-plating (DDP) fixation model for distal radius fractures. Material testing reveals that PLA provides higher rigidity and strength, while PCL offers superior ductility. Mechanical strength tests confirm the superior performance of DU lattice structures under compression, shear, and torsion forces. The bone remodeling program, applied to 36 model combinations of fracture gaps, materials, and lattice designs, computes the total percentage of cell differentiation (TPCD), identifying scaffold material as the key factor, with PLA significantly enhancing TPCD values. Biomechanical analysis after 50 remodeling iterations in a 5.4 mm fracture gap shows that the PLA + DU scaffold reduces displacement by 35%/39%/75%, bone stress by 19%/16%/67%, and fixation plate stress by 77%/66%/93% under axial/bending/torsion loads, respectively, compared to the PCL + YM scaffold. This study highlights the critical role of dynamic remodeling programs in optimizing scaffold material properties and lattice architectures, establishing a robust platform for patient-specific bone repair solutions in regenerative medicine.
Keywords: 3D printing; Biomechanical; Bone remodeling; Lattice; PLA/PCL; Scaffold.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: N/A. Consent for publication: Not applicable. Further disclosure: N/A. Competing interests: The authors declare no competing interests.
Figures








Similar articles
-
3D printed O2-generating scaffolds enhance osteoprogenitor- and type H vessel recruitment during bone healing.Acta Biomater. 2024 Sep 1;185:126-143. doi: 10.1016/j.actbio.2024.07.011. Epub 2024 Jul 14. Acta Biomater. 2024. PMID: 39009209 Free PMC article.
-
Computational optimization of 3D printed bone scaffolds using orthogonal array-driven FEA and neural network modeling.Sci Rep. 2025 Aug 20;15(1):30515. doi: 10.1038/s41598-025-15122-5. Sci Rep. 2025. PMID: 40835669 Free PMC article.
-
Mechanical analysis and sensitivity evaluation of PLA scaffolds for bone tissue repair using FEA and Taguchi experimental design.Acta Bioeng Biomech. 2025 Jun 16;27(1):69-81. doi: 10.37190/abb-02572-2024-03. Print 2025 Mar 1. Acta Bioeng Biomech. 2025. PMID: 40239170
-
Strategic advances in Vat Photopolymerization for 3D printing of calcium phosphate-based bone scaffolds: A review.Bioact Mater. 2025 Jun 27;52:719-752. doi: 10.1016/j.bioactmat.2025.05.001. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40677755 Free PMC article. Review.
-
Bone density and fracture risk factors in ankylosing spondylitis: a meta-analysis.Osteoporos Int. 2024 Jan;35(1):25-40. doi: 10.1007/s00198-023-06925-1. Epub 2023 Oct 9. Osteoporos Int. 2024. PMID: 37814094
References
-
- Li CH, Wu CH, Lin CL. Design of a patient-specific mandible reconstruction implant with dental prosthesis for metal 3D printing using integrated weighted topology optimization and finite element analysis. J Mech Behav Biomed Mater. 2020;105:103700. - PubMed
-
- Ramavath D, Yeole SY, Kode JP, Pothula N, Devana SR. Development of patient-specific 3D printed implants for total knee arthroplasty. Explo Med. 2023;4(6):1033–47.
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
