Immune checkpoint inhibitors in cancer therapy: what lies beyond monoclonal antibodies?
- PMID: 40536609
- PMCID: PMC12178997
- DOI: 10.1007/s12032-025-02822-1
Immune checkpoint inhibitors in cancer therapy: what lies beyond monoclonal antibodies?
Abstract
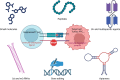
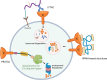
Immune checkpoints are critical in modulating immune responses and maintaining self-tolerance. Cancer cells can exploit these mechanisms to evade immune detection, making immune checkpoints attractive targets for cancer therapy. The introduction of immune checkpoint inhibitors (ICIs) has transformed cancer treatment, with monoclonal antibodies targeting CTLA-4, PD-1, and PD-L1 demonstrating clinical success. However, challenges such as immune-related adverse events, primary and acquired resistance, and high treatment costs persist. To address these challenges, it is essential to explore alternative strategies, including small-molecule and peptide-based inhibitors, aptamers, RNA-based therapies, gene-editing technologies, bispecific and multispecific agents, and cell-based therapies. Additionally, innovative approaches such as lysosome-targeting chimeras, proteolysis-targeting chimeras, and N-(2-hydroxypropyl) methacrylamide copolymers are emerging as promising options for enhancing treatment effectiveness. This review highlights significant advancements in the field, focusing on their clinical implications and successes.
Keywords: Cancer therapy; Immune checkpoint inhibitors; Immunotherapy; Monoclonal antibodies; Small molecules.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures
Similar articles
-
Antibody treatment of hepatocellular carcinoma: a review of current and emerging approaches.Front Immunol. 2025 Jun 20;16:1533874. doi: 10.3389/fimmu.2025.1533874. eCollection 2025. Front Immunol. 2025. PMID: 40621467 Free PMC article. Review.
-
Glioblastoma Immunotherapy: A Systematic Review of the Present Strategies and Prospects for Advancements.Int J Mol Sci. 2023 Oct 10;24(20):15037. doi: 10.3390/ijms242015037. Int J Mol Sci. 2023. PMID: 37894718 Free PMC article.
-
Cancer cell-derived extracellular vesicles: a potential target for overcoming tumor immunotherapy resistance and immune evasion strategies.Front Immunol. 2025 Jun 12;16:1601266. doi: 10.3389/fimmu.2025.1601266. eCollection 2025. Front Immunol. 2025. PMID: 40574844 Free PMC article. Review.
-
Fluorinated small molecule derivatives in cancer immunotherapy: emerging frontiers and therapeutic potential.Front Immunol. 2025 Jul 18;16:1622091. doi: 10.3389/fimmu.2025.1622091. eCollection 2025. Front Immunol. 2025. PMID: 40755757 Free PMC article. Review.
-
Current studies of immunotherapy in head and neck cancer.Clin Otolaryngol. 2018 Feb;43(1):13-21. doi: 10.1111/coa.12895. Epub 2017 May 29. Clin Otolaryngol. 2018. PMID: 28464441
Cited by
-
Clinical application prospects of traditional Chinese medicine as adjuvant therapy for metabolic reprogramming in colorectal cancer.Front Immunol. 2025 Jul 15;16:1630279. doi: 10.3389/fimmu.2025.1630279. eCollection 2025. Front Immunol. 2025. PMID: 40735327 Free PMC article. Review.
References
-
- Li S-J, Sun Z-J. Fueling immune checkpoint blockade with oncolytic viruses: current paradigms and challenges ahead. Cancer Lett. 2022;550: 215937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

