Immune checkpoint inhibitors in cancer therapy: what lies beyond monoclonal antibodies?
- PMID: 40536609
- PMCID: PMC12178997
- DOI: 10.1007/s12032-025-02822-1
Immune checkpoint inhibitors in cancer therapy: what lies beyond monoclonal antibodies?
Abstract
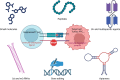
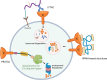
Immune checkpoints are critical in modulating immune responses and maintaining self-tolerance. Cancer cells can exploit these mechanisms to evade immune detection, making immune checkpoints attractive targets for cancer therapy. The introduction of immune checkpoint inhibitors (ICIs) has transformed cancer treatment, with monoclonal antibodies targeting CTLA-4, PD-1, and PD-L1 demonstrating clinical success. However, challenges such as immune-related adverse events, primary and acquired resistance, and high treatment costs persist. To address these challenges, it is essential to explore alternative strategies, including small-molecule and peptide-based inhibitors, aptamers, RNA-based therapies, gene-editing technologies, bispecific and multispecific agents, and cell-based therapies. Additionally, innovative approaches such as lysosome-targeting chimeras, proteolysis-targeting chimeras, and N-(2-hydroxypropyl) methacrylamide copolymers are emerging as promising options for enhancing treatment effectiveness. This review highlights significant advancements in the field, focusing on their clinical implications and successes.
Keywords: Cancer therapy; Immune checkpoint inhibitors; Immunotherapy; Monoclonal antibodies; Small molecules.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures
References
-
- Li S-J, Sun Z-J. Fueling immune checkpoint blockade with oncolytic viruses: current paradigms and challenges ahead. Cancer Lett. 2022;550: 215937. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

