Latest updates on pathogenesis mechanisms and management strategies for cytokine release syndrome, neurotoxicity, and hemophagocytic lymphohistiocytosis related to CAR-T cell therapies
- PMID: 40536724
- PMCID: PMC12283850
- DOI: 10.1007/s00277-025-06467-y
Latest updates on pathogenesis mechanisms and management strategies for cytokine release syndrome, neurotoxicity, and hemophagocytic lymphohistiocytosis related to CAR-T cell therapies
Abstract
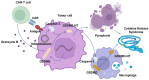
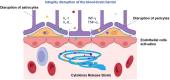
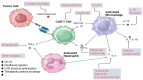
Nowadays, chimeric antigen receptor (CAR) -T cell therapy has shown significant efficacy in treating hematological tumors, with an obvious increase in patient survival rates. However, with the widespread application of CAR-T, the incidence of CAR-T related adverse events has gradually increased, including cytokine release syndrome (CRS), immune effector cell associated neurotoxicity syndrome (ICANS), and hemophagocytic lymphohistiocytosis (HLH). These complications may be life-threatening, so early diagnosis and intervention treatment are crucial for the prognosis of patients. In this review, we first comprehensively summarize the latest insights into the pathogenesis and clinical manifestations of CRS, ICANS, and HLH after CAR-T, with a focus on elaborating on the specific mechanisms and related pathways of the inflammatory storm caused by a large number of cytokines after CAR-T. We also discussed the established prevention and management strategies for the three complications mentioned above, and demonstrated the effectiveness of the treatment by introducing the therapeutic effects of various treatment methods in current clinical or preclinical trials. In addition, we provide a prospective perspective on the measures and modifications currently being studied to mitigate the toxicity associated with CAR-T cell therapy.
Keywords: Chimeric antigen receptor (CAR)-T cell therapies; Cytokine release syndrome; Hemophagocytic lymphohistiocytosis; Immune effector cell-associated neurotoxicity syndrome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics statement: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Riding the storm: managing cytokine-related toxicities in CAR-T cell therapy.Semin Immunopathol. 2024 Jul 16;46(3-4):5. doi: 10.1007/s00281-024-01013-w. Semin Immunopathol. 2024. PMID: 39012374 Free PMC article. Review.
-
[Management of side effects of CAR T cells].Inn Med (Heidelb). 2025 Aug;66(8):818-827. doi: 10.1007/s00108-025-01944-y. Epub 2025 Jul 8. Inn Med (Heidelb). 2025. PMID: 40643658 Free PMC article. Review. German.
-
Current and emerging pharmacotherapies for cytokine release syndrome, neurotoxicity, and hemophagocytic lymphohistiocytosis-like syndrome due to CAR T cell therapy.Expert Opin Pharmacother. 2024 Feb;25(3):263-279. doi: 10.1080/14656566.2024.2340738. Epub 2024 Apr 10. Expert Opin Pharmacother. 2024. PMID: 38588525 Review.
-
Mitigation and Management of Common Toxicities Associated with the Administration of CAR-T Therapies in Oncology Patients.Drug Saf. 2025 Jul;48(7):719-737. doi: 10.1007/s40264-025-01538-5. Epub 2025 Mar 19. Drug Saf. 2025. PMID: 40108072 Free PMC article. Review.
-
Chimeric antigen receptor (CAR) T-cell therapy for people with relapsed or refractory diffuse large B-cell lymphoma.Cochrane Database Syst Rev. 2021 Sep 13;9(9):CD013365. doi: 10.1002/14651858.CD013365.pub2. Cochrane Database Syst Rev. 2021. PMID: 34515338 Free PMC article.
References
-
- Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, Zhang L, Wei G, Tian Y, Zhao K, Chen A, Tan B, Cui J, Li D, Li Y, Qi Y, Wang D, Wu Y, Li D, Du B, Liu M, Huang H (2022) Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature 609(7926):369–374. 10.1038/s41586-022-05140-y - PMC - PubMed
-
- Shi M, Wang J, Huang H, Liu D, Cheng H, Wang X, Chen W, Yan Z, Sang W, Qi K, Li D, Zhu F, Li Z, Qiao J, Wu Q, Zeng L, Fei X, Gu W, Miao Y, Xu K, Zheng J, Cao J (2024) Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun 15(1):3371. 10.1038/s41467-024-47801-8 - PMC - PubMed
-
- Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, Dreger P (2021) Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol 32(1):34–48. 10.1016/j.annonc.2020.10.478 - PubMed
-
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378(5):439–448. 10.1056/NEJMoa1709866 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Grant No. TJWJ2024QN040/Tianjin Health Research Project
- 24ZXGZSY00120/The major special project on public health science and technology in Tianjin
- TJYXZDXK-056B/Tianjin Key Medical Discipline (Specialty) Construction Project
- TJWJ2022XK018, TJWJ2022QN030/the Science and Technology Project of Tianjin Municipal Health Committee
LinkOut - more resources
Full Text Sources

