Obesity and Schizophrenia: Results of a Feasibility Study with Semaglutide to Assist Weight Loss
- PMID: 40536760
- PMCID: PMC12313766
- DOI: 10.1007/s12325-025-03261-0
Obesity and Schizophrenia: Results of a Feasibility Study with Semaglutide to Assist Weight Loss
Abstract
Introduction: Weight gain has come to define the life experience of many individuals with schizophrenia and other severe enduring mental illnesses (SMI). In this clinical intervention study, we aimed to determine whether weekly treatment with the glucagon-like peptide-1 (GLP-1) agonist, semaglutide, as part of usual care, is feasible and acceptable to individuals in a psychiatric inpatient setting.
Methods: Fifteen inpatients (11 men/4 women) in a secure care environment, diagnosed with schizophrenia or schizoaffective disorder and with body mass index (BMI) of at least 30 kg/m2 were commenced on weekly subcutaneous semaglutide as per standard of care. BMI and glycated haemoglobin (HbA1c) were measured at baseline and monthly follow-up to 6 months, and quality of life (QOL) was surveyed at baseline and 6 months. Analysis was based on intention-to-treat.
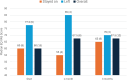
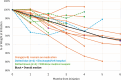
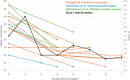
Results: Mean age of patients was 37 years (range 23-63). Time since diagnosis varied from 2 to 25 years. Mean initial BMI was 48.7 kg/m2 for women and 37.2 kg/m2 for men. Duration of semaglutide treatment ranged from 2-6 months. EuroQol 5-Dimensional Questionnaire, 5-Level Version Visual Analogue Scale (EQ5D5L QOL VAS) showed a mean improvement of + 7.5 (from 60 to 67.5) points. Improvement in QOL was overall significantly greater in those who remained on semaglutide (+ 9.5) than those who discontinued. Six patients discontinued semaglutide before the study end, including two who were discharged and no longer able to receive the intervention, and four who withdrew due to medical concerns. Individual percentage weight change varied from + 1 to - 12% (median 5%), and weight reduction was seen in all except two patients. All but one patient demonstrated a reduction in HbA1c levels. Mean HbA1c fell significantly from 41 (range 34-47) mmol/mol to 35.3 (31-45) mmol/mol. Importantly, all patients with baseline HbA1c in the non-diabetic hyperglycaemia range (42-47 mmol/mol) demonstrated a reduction of HbA1c to below 42 mmol/mol by 3 months. Prior to initiation of semaglutide, mean blood pressure was 127 (range 117-145) mmHg systolic and 82 (62-99) mmHg diastolic. At last assessment, average blood pressure was reduced to 121 (107-136) mmHg systolic and 79 (65-96) mmHg diastolic.
Conclusion: In this feasibility study, weekly semaglutide treatment was associated with improvement in self-rated overall QOL and reductions in BMI, HbA1c and blood pressure at up to 6 months follow-up. Even in patients who discontinued treatment before 6 months, initial benefits of weight reduction and improved QOL were still demonstrated. Further evaluation, including health economic assessment and longer-term follow up, may support the expanded use of GLP-1 agonists in improving the cardiometabolic profile and longitudinal health outcomes in individuals with SMI.
Keywords: Feasibility; GLP-1; Obesity; Schizophrenia; Treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Adrian H Heald, Gavin Reynolds, Isabel Nash, Onagh Boyle, Chris Daly, Damien Longson, Donal O’Shea, Joseph Ingram, Richard Holt, Joseph Firth, Mike Stedman, Akheel Syed, Marc de Hert have no conflict of interest. Ethics: This intervention formed part of usual care based on clinical need. Therefore ethical approval was not sought. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013. This was a service improvement project. The UK Health Research Authority decision tool did not consider this study to be research and therefore NHS Research Ethics Committee review was not required. All patients who received semaglutide gave verbal consent to receiving this medication as part of their usual care. Their consent was recorded in the electronic patient hospital record.
Figures
References
-
- Heald AH, Martin JL, Payton T, Khalid L, Anderson SG, Narayanan RP, De Hert M, Yung A, Livingston M. Changes in metabolic parameters in patients with severe mental illness over a 10-year period: a retrospective cohort study. Aust N Z J Psychiatry. 2017;51:75–82. - PubMed
-
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686–96. - PubMed
-
- Cooper SJ, Reynolds GP; With expert co-authors (in alphabetical order): Barnes T, England E, Haddad PM, Heald A, Holt R, Lingford-Hughes A, Osborn D, McGowan O, Patel MX, Paton C, Reid P, Shiers D, Smith J. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2016;30:717–48. - PubMed
-
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiat. 2014;71:1350–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

