Insights From Amniotic and Umbilical Cord Mesenchymal Stem Cells in Wound Healing
- PMID: 40536887
- PMCID: PMC12178269
- DOI: 10.1111/jcmm.70679
Insights From Amniotic and Umbilical Cord Mesenchymal Stem Cells in Wound Healing
Abstract
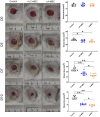
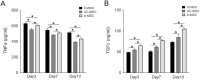
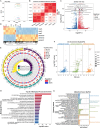
Skin repair is a complex physiological process that involves the coordinated actions of various cell types. This study examines the distinct roles of amniotic mesenchymal stem cells (A-MSCs) and umbilical cord mesenchymal stem cells (UC-MSCs) in skin healing using a mouse model. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed significant differences in gene expression between A-MSCs and UC-MSCs. Specifically, A-MSCs exhibited upregulation of genes associated with extracellular matrix (ECM) organisation and cell migration, thereby enhancing their tissue remodelling capabilities. In contrast, UC-MSCs demonstrate increased expression of genes involved in angiogenesis and anti-inflammatory responses, highlighting their role in creating a favourable healing environment. These findings highlight the unique therapeutic potentials of A-MSCs and UC-MSCs in skin repair strategies. Although MSCs hold promise in regenerative medicine, challenges such as optimal cell selection and modulation of the inflammatory microenvironment remain to be addressed. Our research emphasises the need for continued research related to properties of MSCs to refine therapeutic approaches for effective wound healing.
Keywords: amniotic membrane; inflammatory microenvironment; mesenchymal stem cell; skin repair; umbilical cord.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Human umbilical cord mesenchymal stem cell exosomes promote elastin production and acute skin wound healing via TGFβ1-Smad pathway.Mol Cell Biochem. 2025 Jul;480(7):4499-4511. doi: 10.1007/s11010-025-05264-5. Epub 2025 Apr 9. Mol Cell Biochem. 2025. PMID: 40202710 Free PMC article.
-
Platelet-rich plasma enhances local homing of umbilical cord-derived mesenchymal stem cells to articular cartilage by increasing the quantity and activation of integrin ꞵ1.Stem Cell Res Ther. 2025 Sep 2;16(1):487. doi: 10.1186/s13287-025-04593-y. Stem Cell Res Ther. 2025. PMID: 40898269 Free PMC article.
-
Mesenchymal stem cell secretome alters gene expression and upregulates motility of human endometrial stromal cells.Reproduction. 2023 Jul 5;166(2):161-174. doi: 10.1530/REP-22-0485. Print 2023 Aug 1. Reproduction. 2023. PMID: 37252830 Free PMC article.
-
The Hidden Power of the Secretome: Therapeutic Potential on Wound Healing and Cell-Free Regenerative Medicine-A Systematic Review.Int J Mol Sci. 2025 Feb 23;26(5):1926. doi: 10.3390/ijms26051926. Int J Mol Sci. 2025. PMID: 40076553 Free PMC article.
-
Insights into the role of mesenchymal stem cells in cutaneous medical aesthetics: from basics to clinics.Stem Cell Res Ther. 2024 Jun 18;15(1):169. doi: 10.1186/s13287-024-03774-5. Stem Cell Res Ther. 2024. PMID: 38886773 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

