Pericyte-glial cell interactions: Insights into brain health and disease
- PMID: 40537003
- PMCID: PMC12407509
- DOI: 10.4103/NRR.NRR-D-24-01472
Pericyte-glial cell interactions: Insights into brain health and disease
Abstract
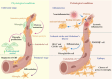
Pericytes are multi-functional mural cells of the central nervous system that cover the capillary endothelial cells. Pericytes play a vital role in nervous system development, significantly influencing the formation, maturation, and maintenance of the central nervous system. An expanding body of studies has revealed that pericytes establish carefully regulated interactions with oligodendrocytes, microglia, and astrocytes. These communications govern numerous critical brain processes, including angiogenesis, neurovascular unit homeostasis, blood-brain barrier integrity, cerebral blood flow regulation, and immune response initiation. Glial cells and pericytes participate in dynamic and reciprocal interactions, with each influencing and adjusting the functionality of the other. Pericytes have the ability to control astrocyte polarization, trigger differentiation of oligodendrocyte precursor cells, and initiate immunological responses in microglia. Various neurological disorders that compromise the integrity of the blood-brain barrier can disrupt these communications, impair waste clearance, and hinder cerebral blood circulation, contributing to neuroinflammation. In the context of neurodegeneration, these disruptions exacerbate pathological processes, such as neuronal damage, synaptic dysfunction, and impaired tissue repair. This article explores the complex interactions between pericytes and various glial cells in both healthy and pathological states of the central nervous system. It highlights their essential roles in neurovascular function and disease progression, providing important insights that may enhance our understanding of the molecular mechanisms underlying these interactions and guide potential therapeutic strategies for neurodegenerative disorders in future research.
Keywords: brain; inflammation; neuroprotection; neurovascular function; therapeutic targets.
Copyright © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures



References
-
- Alarcon-Martinez L, Yemisci M, Dalkara T. Pericyte morphology and function. Histol Histopathol. 2021;36:633–643. - PubMed
-
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci U S A. 2003;100:2106–2111. - PMC - PubMed
LinkOut - more resources
Full Text Sources

