Stem cell repair strategies for epilepsy
- PMID: 40537022
- PMCID: PMC12407534
- DOI: 10.4103/NRR.NRR-D-24-01337
Stem cell repair strategies for epilepsy
Abstract
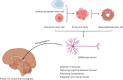
Epilepsy is a serious neurological disorder; however, the effectiveness of current medications is often suboptimal. Recently, stem cell technology has demonstrated remarkable therapeutic potential in addressing various neurological diseases, igniting interest in its applicability for epilepsy treatment. This comprehensive review summarizes different therapeutic approaches utilizing various types of stem cells. Preclinical experiments have explored the use and potential therapeutic effects of mesenchymal stem cells, including genetically modified variants. Clinical trials involving patient-derived mesenchymal stem cells have shown promising results, with reductions in the frequency of epileptic seizures and improvements in neurological, cognitive, and motor functions reported. Another promising therapeutic strategy involves neural stem cells. These cells can be cultured outside the body and directed to differentiate into specific cell types. The transplant of neural stem cells has the potential to replace lost inhibitory interneurons, providing a novel treatment avenue for epilepsy. Embryonic stem cells are characterized by their significant capacity for self-renewal and their ability to differentiate into any type of somatic cell. In epilepsy treatment, embryonic stem cells can serve three primary functions: neuron regeneration, the maintenance of cellular homeostasis, and restorative activity. One notable strategy involves differentiating embryonic stem cells into γ-aminobutyric acidergic neurons for transplantation into lesion sites. This approach is currently undergoing clinical trials and could be a breakthrough in the treatment of refractory epilepsy. Induced pluripotent stem cells share the same genetic background as the donor, thereby reducing the risk of immune rejection and addressing ethical concerns. However, research on induced pluripotent stem cell therapy remains in the preclinical stage. Despite the promise of stem cell therapies for epilepsy, several limitations must be addressed. Safety concerns persist, including issues such as tumor formation, and the low survival rate of transplanted cells remains a significant challenge. Additionally, the high cost of these treatments may be prohibitive for some patients. In summary, stem cell therapy shows considerable promise in managing epilepsy, but further research is needed to overcome its existing limitations and enhance its clinical applicability.
Keywords: astrocyte transdifferentiation; cell therapy; cell transplantation; clinical trials; embryonic pluripotent stem cells; epilepsy; gamma-aminobutyric acidergic neuron; induced pluripotent stem cells; mesenchymal stem cells; nerve regeneration; neural stem cells; organoid.
Copyright © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures




References
-
- Ababneh NA, Scaber J, Flynn R, Douglas A, Barbagallo P, Candalija A, Turner MR, Sims D, Dafinca R, Cowley SA, Talbot K. Correction of amyotrophic lateral sclerosis related phenotypes in induced pluripotent stem cell-derived motor neurons carrying a hexanucleotide expansion mutation in C9orf72 by CRISPR/Cas9 genome editing using homology-directed repair. Hum Mol Genet. 2020;29:2200–2217. - PMC - PubMed
-
- Adeyeye A, Mirsadeghi S, Gutierrez M, Hsieh J. Integrating adult neurogenesis and human brain organoid models to advance epilepsy and associated behavioral research. Epilepsy Behav. 2024;159:109982. - PubMed
LinkOut - more resources
Full Text Sources

