Acute and Long-Term Immune-Treatment Strategies in Anti-LGI1 Antibody-Mediated Encephalitis: A Multicenter Cohort Study
- PMID: 40537079
- PMCID: PMC12185223
- DOI: 10.1212/NXI.0000000000200412
Acute and Long-Term Immune-Treatment Strategies in Anti-LGI1 Antibody-Mediated Encephalitis: A Multicenter Cohort Study
Abstract
Background and objectives: Few studies have evaluated acute immunotherapy and relapse prevention strategies in patients with anti-leucine-rich glioma-inactivated 1 (LGI1) antibody (Ab)-mediated encephalitis. The objective of this study was to analyze the outcomes of acute and long-term immunotherapy strategies in this population.
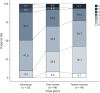
Methods: We undertook a multicenter cohort study of 55 patients with anti-LGI1 Ab-mediated encephalitis, either recruited prospectively or identified retrospectively from 10 Australian hospitals as part of the Australian Autoimmune Encephalitis Consortium. Clinical data were collected, including treatment durations of all relevant immunotherapies. Clinical outcomes that we examined included (1) time to first clinical relapse, (2) improvement on modified Rankin Scale (mRS), and (3) favorable binary composite clinical-functional outcome at 12 months. A favorable outcome was defined as fulfilling all three of mRS less than 3, a score of 1 or less in the memory dysfunction component of the Clinical Assessment Scale in Autoimmune Encephalitis, and absence of drug-resistant epilepsy.
Results: Rituximab, adjusted for concomitant use of other immunotherapies, was associated with increased time to first relapse (hazard ratio 0.10; 95% CI 0.001-0.85; p = 0.03). Intravenous pulsed methylprednisolone was associated with an improvement in mRS (OR 4.48; 95% CI 1.03-21.3; p = 0.048) and a favorable composite clinical-functional outcome (OR 4.96; 95% CI 1.07-27.2; p = 0.049) at 12 months.
Discussion: Rituximab may be effective at preventing relapses in patients with anti-LGI1 Ab-mediated encephalitis. Acute methylprednisolone treatment may be associated with favorable outcomes at 12 months.
Classification of evidence: This study provides Class IV evidence that for patients with anti-LGI1 Ab-mediated encephalitis, rituximab prevents relapses and acute methylprednisolone is associated with favorable outcomes at 12 months.
Conflict of interest statement
Stephen Blum is and has been involved in clinical trials sponsored by Roche, Novartis, Sanofi-Genzyme, CSL, Clene Nanomedicine, Biogen and Merck. His institution have received honoraria for advisory boards and speaking honoraria from Biogen, Merck, Roche and Novartis; H. Butzkueven is an employee of Monash University and has accepted travel compensation from Merck; his institution receives honoraria for talks, steering committee activities, and research grants from Roche, Merck, Biogen, Novartis, and UCB Pharma, Medical Research Future Fund Australia, National Health and Medical Research Council (NHMRC) Australia, Trish MS Foundation, MS Australia and the Pennycook Foundation. He receives personal compensation for steering group activities for the Brain Health Initiative from Oxford Health Policy Forum and is funded by an NHMRC Australia Investigator Grant; Katherine Buzzard is principal investigator in clinical trials for Novartis, Merck, Roche and Biogen. She has received speakers honorarium and/or travel grants from Sanofi Genzyme, Roche, Alexion, Merck, Biogen, Novartis and Teva. She has been on advisory boards for Merck, Biogen and UCB; Todd Hardy has received honoraria for talks, advisory boards or support for scientific meetings from Bayer-Schering, Novartis, Biogen Idec, Merck, Teva, Merck, Alexion, Bristol Myers Squibb and Sanofi-Genzyme. He has been the principal investigator on the phase IV trials in MS funded by Novartis and Sanofi Genzyme. He is Co-Editor of Advances in Clinical Neuroscience and Rehabilitation and serves on the editorial boards of Journal of Neuroimmunology and Frontiers in Neurology; Tomas Kalincik served on scientific advisory boards for MS International Federation and World Health Organisation, BMS, Roche, Janssen, Sanofi Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Roche, Sanofi-Genzyme, Teva, BioCSL and Merck and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck; Richard Macdonell or his institution have received remuneration for his speaking engagements, advisory board memberships, research and travel from Biogen, Merck, Sanofi-Genzyme, Bayer, Roche, Teva, Novartis, CSL, BMS, MedDay and NHMRC; Laurie McLaughlin - has received educational event support from Roche, Biogen and Merck; M. Monif has served on the advisory board for Merck and Novartis, and has received speaker honoraria from Merck, Biogen and Novartis. Her institution receives funding from Merck, Australian National Health Medical Research Council, and MS Research Australia; C. Nesbitt has received speaker honoraria and or educational travel support from Merck and Biogen and Roche; S. Ramanathan has received research funding from the NHMRC (Australia), the Petre Foundation, the Brain Foundation, the Royal Australasian College of Physicians, and the University of Sydney. She is supported by an NHMRC Investigator Grant (GNT2008339). She serves as a consultant on an advisory board for UCB and Limbic Neurology, and has been an invited speaker for educational/research sessions coordinated by Biogen, Alexion, Novartis, Excemed and Limbic Neurology. She is on the medical advisory board (non-remunerated positions) of The MOG Project and the Sumaira Foundation; S. Reddel Funds over the last 5 years including but not limited to travel support, honoraria, trial payments, research and clinical support to the neurology department or academic projects of which The authors am a member has been received from bodies and charities: NHMRC, MRFF, NBA, Myasthenia Alliance Australia, Beeren foundation; and from pharmaceutical/biological companies: Alexion, Biogen, CSL, Genzyme, Grifols, Merck, Novartis, Roche, Sandoz, Sanofi, UCB. Additional interests and potential conflicts of interest include: Co-founder/shareholder of RxPx health, National IVIG Governance Advisory Council and Specialist Working Group Australia (Neurology) (paid), Australian Medical Services Advisory Committee ad hoc sub-committee on IVIG (paid), Australian Technical Advisory Group on Immunisation Varicella Zoster working party (unpaid), Public Salary as a staff specialist neurologist from Concord Hospital Sydney Local Health District (paid), Private billings from patients and medicare Australia reimbursement as a private practice neurologist (paid), Medical advisor (unpaid) to various patient and advocacy groups; Nabil Seery has received conference fee sponsorship from Roche; T. Tan–has received speaker honoraria from Eisai and travel support from Biogen; R. Wesselingh–nothing to declare The other authors declare no competing interests. Full disclosure form information provided by the authors is available with the full text of this article at
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials