Good Ethical and Laboratory Practices for Wastewater Surveillance
- PMID: 40537440
- PMCID: PMC12178841
- DOI: 10.1002/wer.70112
Good Ethical and Laboratory Practices for Wastewater Surveillance
Abstract
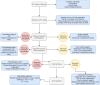
Wastewater Surveillance (WBS) is an approach for disease surveillance involving the screening of wastewater for RNA/DNA originating from infectious agents. In recent years, WBS has expanded to include analytes from pharmaceuticals (such as SSRI) or illicit drugs, referred to as "High-risk Substances" (HRS). The University of Maryland enacted in partnership with a local county public health department and water utility, a surveillance program to assess wastewater twice weekly for the presence of SARS-CoV-2 and other viral targets. WBS can provide rapid data showing where the location of clustered outbreaks may be occurring (specifically. The county public health department also requested that screening of high-risk substances (fentanyl, ketamine, Narcan, heroine, etc.) to be included in WBS, thus expanding the purview of the initial surveillance project focusing solely biological agents. A concern for any surveillance program is adhering to ethical standards, regulations, and protocols. In WBS, there is no single standardized "list of rules" to guide researchers in determining risk of privacy or community stigmatization. In an effort to counter the variation in WBS as it pertains to ethical standards, we propose utilizing an ethical scoring framework tailored for WBS. This framework includes a scoresheet that can assist scientists determine the privacy and ethical risks associated with their study by introducing a quantifiable process to assess ethical compliance. Furthermore, we include a flow map outlining standard laboratory practices under the lens of how each step assists in maintaining sample fidelity, thus increasing the robustness and reliability of the data generated. SUMMARY: It is important to balance ethical standards, public health and research strategies. Application of the tools listed in this document will ensure this balance. Application of the developed ethical guidelines (Score sheet) is a practical approach. It is important to integrate vigorous data management practices. Utilization of a robust and holistic structure of the wastewater surveillance program will ensure successful outcomes.
© 2025 The Author(s). Water Environment Research published by Wiley Periodicals LLC on behalf of Water Environment Federation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Interventions targeted at women to encourage the uptake of cervical screening.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD002834. doi: 10.1002/14651858.CD002834.pub3. Cochrane Database Syst Rev. 2021. PMID: 34694000 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
References
-
- 2007. ACLU of Arizona Sues County Officials Over Inhumane Confinement of TB Patient. American Civil Liberties Union. https://www.aclu.org/press‐releases/aclu‐arizona‐sues‐county‐officials‐o....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

