Diffusion Tensor Imaging Findings in Cerebral Sensorimotor Areas in Patients After Spinal Cord Injury Correlate With Neurophysiological Deficits
- PMID: 40537462
- PMCID: PMC12405646
- DOI: 10.1177/15459683251345434
Diffusion Tensor Imaging Findings in Cerebral Sensorimotor Areas in Patients After Spinal Cord Injury Correlate With Neurophysiological Deficits
Abstract
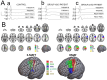
ObjectivesAssessment of sensorimotor cortex and tracts degeneration using novel diffusion tensor imaging (DTI) templates in patients with chronic spinal cord injury (SCI) and its correlation with clinical and neurophysiological findings.MethodsSex and age-matched 29 patients with chronic SCI (paraplegic: p-SCI; tetraplegic: t-SCI) and 29 healthy controls underwent neurophysiological assessment including motor evoked potentials (MEP). DTI was performed on 3 T magnetic resonance imaging scanner and postprocessed using Human Motor Area and Sensorimotor Area Tract Templates. DTI parameters were compared using analysis of covariance with post hoc Scheffé and Bonferroni corrections. Spearman's rank test was used for correlations with P < .05 considered significant.ResultsCompared to controls, all SCI patients showed significantly lower fractional anisotropy (FA) in several tracts (primary motor [M1], somatosensory [S1], pre-supplementary motor area [preSMA], and dorsal premotor [PMd]) and cortices (M1, pre-SMA, and S1). There were no differences in DTI parameters between p-SCI and t-SCI or p-SCI and controls. Compared to controls, t-SCI showed significantly decreased FA within M1 and S1 tracts. In t-SCI higher motor scores were associated with higher FA from ventral premotor area (PMv) tracts and cortex; higher sensory scores were associated with higher FA from S1 tracts. Positive correlations were found between MEP amplitudes from rectus femoris muscles and FA for M1, PMd, PMv, pre-SMA, SMA tracts, and PMv cortex.ConclusionsDTI shows remote degeneration of sensorimotor cortex and supraspinal tracts in SCI correlating with several clinical motor and sensory scores, and MEP parameters. DTI metrics have the potential to become biomarkers of remote degeneration.
Keywords: chronic spinal cord injury; clinical metrics; diffusion tensor imaging; motor evoked potentials; sensorimotor cortex; sensorimotor tracts.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Huber J, Leszczyńska K, Wincek A, et al. The role of peripheral nerve electrotherapy in functional recovery of muscle motor units in patients after incomplete spinal cord injury. Appl Sci. 2021;11(20):9764. doi: 10.3390/app11209764 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

