TGFα controls checkpoints in CNS resident and infiltrating immune cells to promote resolution of inflammation
- PMID: 40537468
- PMCID: PMC12179293
- DOI: 10.1038/s41467-025-60363-7
TGFα controls checkpoints in CNS resident and infiltrating immune cells to promote resolution of inflammation
Abstract
After acute lesions in the central nervous system (CNS), the interaction of microglia, astrocytes, and infiltrating immune cells decides over their resolution or chronification. However, this CNS-intrinsic cross-talk is poorly characterized. Analyzing cerebrospinal fluid (CSF) samples of Multiple Sclerosis (MS) patients as well as CNS samples of female mice with experimental autoimmune encephalomyelitis (EAE), the animal model of MS, we identify microglia-derived TGFα as key factor driving recovery. Through mechanistic in vitro studies, in vivo treatment paradigms, scRNA sequencing, CRISPR-Cas9 genetic perturbation models and MRI in the EAE model, we show that together with other glial and non-glial cells, microglia secrete TGFα in a highly regulated temporospatial manner in EAE. Here, TGFα contributes to recovery by decreasing infiltrating T cells, pro-inflammatory myeloid cells, oligodendrocyte loss, demyelination, axonal damage and neuron loss even at late disease stages. In a therapeutic approach in EAE, blood-brain barrier penetrating intranasal application of TGFα attenuates pro-inflammatory signaling in astrocytes and CNS infiltrating immune cells while promoting neuronal survival and lesion resolution. Together, microglia-derived TGFα is an important mediator of glial-immune crosstalk, highlighting its therapeutic potential in resolving acute CNS inflammation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All authors declare no competing interests.
Figures

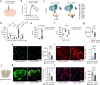

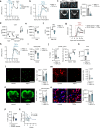
References
-
- Zheng, B. & Tuszynski, M. H. Regulation of axonal regeneration after mammalian spinal cord injury. Nat. Rev. Mol. Cell Biol.24, 396–413 (2023). - PubMed
-
- Meinl, E., Krumbholz, M., Derfuss, T., Junker, A. & Hohlfeld, R. Compartmentalization of inflammation in the CNS: A major mechanism driving progressive multiple sclerosis. J. Neurol. Sci.274, 42–44 (2008). - PubMed
MeSH terms
Substances
Grants and funding
- R01 ES032323/ES/NIEHS NIH HHS/United States
- 851693/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- 408885537-TRR274/Deutsche Forschungsgemeinschaft (German Research Foundation)
- R01 AI126880/AI/NIAID NIH HHS/United States
- 505539112/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Medical

