G0S2: a potential target for NSCLC identified through prognostic models from multi-Omic analysis of regulatory T cell metabolic genes
- PMID: 40537497
- PMCID: PMC12179314
- DOI: 10.1038/s41698-025-00995-6
G0S2: a potential target for NSCLC identified through prognostic models from multi-Omic analysis of regulatory T cell metabolic genes
Abstract
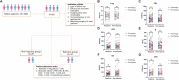
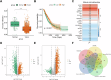
Non-small cell lung cancer (NSCLC) remains a leading cause of cancer-related mortality, with immunotherapy proving effective only in a subset of patients, highlighting the urgent need for improved biomarkers and therapeutic targets. This study explored regulatory T cell (Treg) metabolism-related genes in NSCLC and established a seven-gene prognostic signature (TIMP1, BIRC3, G0S2, PRKCB, PDE4B, CD52, and ACP5) through multi-omics analysis. The model stratified patients into high- and low-risk groups exhibiting distinct immune profiles and drug sensitivities. Clinical validation confirmed that elevated BIRC3 and G0S2 expression correlated with poorer prognosis, while functional assays demonstrated that G0S2 inhibition suppressed tumour progression and reduced Treg infiltration in vivo. These findings position G0S2 as a promising biomarker for immunotherapy response and a potential therapeutic target, providing insights into Treg-mediated immune regulation and advancing personalized NSCLC treatment strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests. Consent for publication: All authors agreed to publish this manuscript.
Figures






Similar articles
-
Identification and validation of a KRAS-macrophage-associated gene signature as prognostic biomarkers and potential therapeutic targets in melanoma.Front Immunol. 2025 Jun 18;16:1566432. doi: 10.3389/fimmu.2025.1566432. eCollection 2025. Front Immunol. 2025. PMID: 40607411 Free PMC article.
-
Unraveling the role of GPCR signaling in metabolic reprogramming and immune microenvironment of lung adenocarcinoma: a multi-omics study with experimental validation.Front Immunol. 2025 Jun 6;16:1606125. doi: 10.3389/fimmu.2025.1606125. eCollection 2025. Front Immunol. 2025. PMID: 40547013 Free PMC article.
-
[Ferroptosis-related long non-coding RNA to predict the clinical outcome of non-small cell lung cancer after radiotherapy].Beijing Da Xue Xue Bao Yi Xue Ban. 2025 Jun 18;57(3):569-577. doi: 10.19723/j.issn.1671-167X.2025.03.022. Beijing Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40509836 Free PMC article. Chinese.
-
Immunotherapy (excluding checkpoint inhibitors) for stage I to III non-small cell lung cancer treated with surgery or radiotherapy with curative intent.Cochrane Database Syst Rev. 2017 Dec 16;12(12):CD011300. doi: 10.1002/14651858.CD011300.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Dec 6;12:CD011300. doi: 10.1002/14651858.CD011300.pub3. PMID: 29247502 Free PMC article. Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin.70, 7–30 (2020). - PubMed
-
- Duma, N., Santana-Davila, R. & Molina, J. R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc.94, 1623–1640 (2019). - PubMed
-
- Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov.19, 39–56 (2020). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

