Prospective randomized, placebo-controlled study: role of branched-chain amino acids infusion as adjunct therapy post-liver surgery for patients in the intensive care unit
- PMID: 40537743
- PMCID: PMC12180149
- DOI: 10.1186/s12876-025-03696-3
Prospective randomized, placebo-controlled study: role of branched-chain amino acids infusion as adjunct therapy post-liver surgery for patients in the intensive care unit
Abstract
Background and aim: Several animal studies have shown that Branched-chain amino acids (BCAAs) may prevent acute liver injury, although its effects in humans are as yet undetermined. Thus the purpose of this study is to evaluate the impact of intravenous BCAAs infusion on liver profile post-liver surgery in the intensive care unit (ICU).
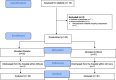
Methods: A randomized study that was applied for post liver surgery patients who were randomly allocated to receive either intravenous BCAA immediately post-operative or placebo.
Measurements: Follow-up liver profile, Child-Pugh, and SOFA scores during the first week post-surgery.
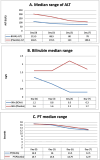
Main results: A significant decline of bilirubin and ALT on day three and five in the BCAA group compared to the control group respectively. There was a significant improvement of PT on day seven 12.5 in the BCAA group versus 12.9 in the control group, p-value 0.01. Total bilirubin levels decreased by 75% in the BCAA group, whereas in the control group saw an increase of 6.25% from the baseline which was statistically significant, p-value 0.0376. SOFA score was markedly improved in the BCAA group (p-value 0.013). In addition to a significantly shorter ICU stay in the BCAA group than in the control group (p-value 0.018).
Conclusion: There are beneficial effects of BCAAs infusion post-liver surgery; including improved metabolic profile (liver function tests), and shorter ICU stay.
Trial registration: (Clinicaltrials.gov registration number:NCT03448848), 28/02/2018.
Keywords: Branched chain amino acid infusion; Critically ill; Hepatic patients; Liver surgery.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol was approved by the institutional review board of the national hepatology and tropical medicine research institute (NHTMRI), Cairo, Egypt. All study procedures were performed in accordance with good clinical practice and the declaration of Helsinki. Written informed consent was obtained from each patient or a legally authorized representative prior to enrollment in the study. The patients' information is kept confidential and not to be viewed except by those who are conducting the research. All patients have the right to refuse to continue the study at any time or not to be included from the start. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Nutritional support for liver disease.Cochrane Database Syst Rev. 2012 May 16;2012(5):CD008344. doi: 10.1002/14651858.CD008344.pub2. Cochrane Database Syst Rev. 2012. PMID: 22592729 Free PMC article.
-
Head Injury Treatment With Healthy and Advanced Dietary Supplements: A Pilot Randomized Controlled Trial of the Tolerability, Safety, and Efficacy of Branched Chain Amino Acids in the Treatment of Concussion in Adolescents and Young Adults.J Neurotrauma. 2024 Jun;41(11-12):1299-1309. doi: 10.1089/neu.2023.0433. Epub 2024 Apr 11. J Neurotrauma. 2024. PMID: 38468511 Free PMC article. Clinical Trial.
-
Magnesium sulfate for acute exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2022 May 26;5(5):CD013506. doi: 10.1002/14651858.CD013506.pub2. Cochrane Database Syst Rev. 2022. PMID: 35616126 Free PMC article.
-
Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit.Cochrane Database Syst Rev. 2018 Mar 27;3(3):CD010754. doi: 10.1002/14651858.CD010754.pub2. Cochrane Database Syst Rev. 2018. PMID: 29582429 Free PMC article.
References
-
- Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54. - PubMed
-
- Tischler ME, Desautels M, Goldberg AL. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982;257(4):1613–21. - PubMed
-
- Higuchi N, Kato M, Masayuki M, et al. Potential role of branched chain amino acids in glucose metabolism through the accelerated induction of the glucose-sensing apparatus in the liver. J Cell Biochemistr. 2010;112(1):30–8. 10.1002/jcb.22688. - PubMed
-
- Muto Y, Sato S, Watanabe A, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3(7):705–13. - PubMed
-
- Moriwaki H, Miwa Y, Tajika M, et al. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313(2):405–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical