The phosphatase PPM1F, a negative regulator of integrin activity, is essential for embryonic development and controls tumor cell invasion
- PMID: 40537771
- PMCID: PMC12180154
- DOI: 10.1186/s12915-025-02254-3
The phosphatase PPM1F, a negative regulator of integrin activity, is essential for embryonic development and controls tumor cell invasion
Abstract
Background: The Mn2+/Mg2+-dependent Ser/Thr phosphatase PPM1F was identified to control integrin activity. Furthermore, PPM1F regulates several protein kinases known to be involved in organizing the cytoskeleton and other cellular functions. Therefore, PPM1F appears critical for a multitude of physiological processes.
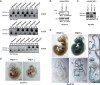
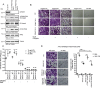
Results: Here, we report the phenotype of ppm1f gene disruption in mice. While heterozygous ppm1f ± mice are viable and fertile, ppm1f-/- mice show severe defects and significant morphological abnormalities in the developing brain and vasculature and abort embryonic development at day E10.5. Isolated ppm1f-/- MEFs or PPM1F-depleted human neuro-epithelial cells display enhanced integrin-dependent cell adhesion, deregulated PAK phosphorylation, and perturbed cell migration. These phenotypes were reversed by re-expression of the wildtype enzyme, but not the phosphatase-inactive PPM1F. In different human tumor cell types, PPM1F expression levels directly correlated with invasive potential, while deletion of PPM1F abrogates tissue invasion.
Conclusions: These results highlight the non-redundant role of this enzyme in integrin and PAK regulation and identify PPM1F as a promising target to limit tumor metastasis.
Keywords: Cancer cell invasion; Cell adhesion; Developmental defects; FilaminA; Integrin activity; Knock-out mouse; PPM1F; Protein phosphatase; Talin; Threonine phosphorylation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Mice were kept in accordance with relevant institutional and national guidelines and regulations in the central animal care facility of University of Konstanz. Procurement of mouse tissues was in accordance with the animal welfare guidelines 2010/63/EC of the European Communities Council Directive and was registered with official authorities (Regierungspräsidium Freiburg, Germany). Consent for publication: Not applicable. Competing interests: The authors declare having no competing interests.
Figures







Similar articles
-
Lockdown, a selective small-molecule inhibitor of the integrin phosphatase PPM1F, blocks cancer cell invasion.Cell Chem Biol. 2022 Jun 16;29(6):930-946.e9. doi: 10.1016/j.chembiol.2022.03.011. Epub 2022 Apr 19. Cell Chem Biol. 2022. PMID: 35443151
-
PPM1F controls integrin activity via a conserved phospho-switch.J Cell Biol. 2020 Dec 7;219(12):e202001057. doi: 10.1083/jcb.202001057. J Cell Biol. 2020. PMID: 33119040 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. - PubMed
-
- Winograd-Katz SE, Fassler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014;15(4):273–88. - PubMed
-
- Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296(5565):151–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

