Intelligent ROS therapy driven by iron-based nanozyme with controllable catalytic activity for infected wound healing
- PMID: 40537805
- PMCID: PMC12178039
- DOI: 10.1186/s12951-025-03495-8
Intelligent ROS therapy driven by iron-based nanozyme with controllable catalytic activity for infected wound healing
Abstract
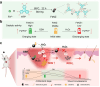
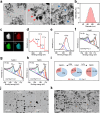
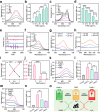
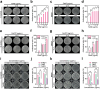
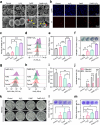
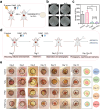
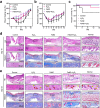
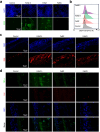
Therapeutic generation of reactive oxygen species (ROS) through catalytic therapy demonstrates antibacterial efficacy against wound infections. However, prolonged and unregulated ROS production risks inducing intolerable oxidative stress alongside exacerbated inflammatory responses, creating a microenvironment counterproductive to wound healing. Here, inspired by rechargeable batteries, we have developed a catalytic activity-controllable nanozyme by integrating Fe(II) and Fe(III) within metal-organic frameworks (FeNZ). Specifically, the overexpressed glutathione in the infective wound can increase the Fe(II) fraction in FeNZ and endow FeNZ with peroxidase (POD)-like activity, which can convert hydrogen peroxide (H2O2) into hydroxyl radicals (•OH) for effective eradication of both drug-sensitive and drug-resistant bacteria (Staphylococcus aureus, 97.9% of antibacterial rate; methicillin-resistant S. aureus (MRSA), 93.2% of antibacterial rate) by disrupting bacterial membranes. Of note, the catalytic performance of FeNZ declined in parallel with the increase in Fe(III) content during the •OH generation process, resulting in a low inflammatory microenvironment for infected wound healing and faster wound healing (95.5% of healing rate for FeNZ + H2O2 group, 83.5% of healing rate for Control group, day 16). The activity-controllable FeNZ thus holds promise as an effective agent for bacterial elimination and enhanced wound repair, presenting a novel strategy for the management of infected wounds.
Keywords: Adjustable enzyme activity; Bacterial infection; Chemodynamic therapy; Nanozyme; Wound repair.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal experiments were reviewed and approved by the Animal Care and Use Committee of Huazhong University of Science and Technology (IACUC Number: 3292). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Metal-Organic Framework-Based Self-Powered Chemodynamic Therapy Nanosystem: CaO2@PCN-222(Fe) and Its Mixed Matrix Membranes for Promoting Infected Wound Healing.Inorg Chem. 2025 Jul 7;64(26):13557-13568. doi: 10.1021/acs.inorgchem.5c02508. Epub 2025 Jun 24. Inorg Chem. 2025. PMID: 40555694
-
Multi-enzymatic biomimetic cerium-based MOFs mediated precision chemodynamic synergistic antibacteria and tissue repair for MRSA-infected wounds.J Nanobiotechnology. 2025 May 20;23(1):364. doi: 10.1186/s12951-025-03349-3. J Nanobiotechnology. 2025. PMID: 40394650 Free PMC article.
-
Promoting the healing of infected diabetic wound by nanozyme-containing hydrogel with anti-bacterial inflammation suppressing, ROS-scavenging and oxygen-generating properties.J Biomed Mater Res B Appl Biomater. 2024 Aug;112(8):e35458. doi: 10.1002/jbm.b.35458. J Biomed Mater Res B Appl Biomater. 2024. PMID: 39122663
-
Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis.Cochrane Database Syst Rev. 2018 Jul 21;7(7):CD009650. doi: 10.1002/14651858.CD009650.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Dec 13;12:CD009650. doi: 10.1002/14651858.CD009650.pub5. PMID: 30030966 Free PMC article. Updated.
-
Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis.Cochrane Database Syst Rev. 2022 Dec 13;12(12):CD009650. doi: 10.1002/14651858.CD009650.pub5. Cochrane Database Syst Rev. 2022. PMID: 36511181 Free PMC article.
References
-
- Organization WH. The top 10 causes of death. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
-
- Brdová D, Ruml T, Viktorová J. Mechanism of Staphylococcal resistance to clinically relevant antibiotics. Drug Resist Updat. 2024;77:101147. - PubMed
MeSH terms
Substances
Grants and funding
- 82072068/National Natural Science Foundation of China
- 82173315/National Natural Science Foundation of China
- 82072167/National Natural Science Foundation of China
- 2024AFA053/Hubei Province Natural Science Foundation
- 2022020801010461/Knowledge Innovation Special Project for Fundamental Research of Wuhan
LinkOut - more resources
Full Text Sources
Medical

