Hope for OTHERS (Our Tissue Helping Enhance Research & Science): research results from the University of Pittsburgh rapid autopsy program for breast cancer
- PMID: 40537829
- PMCID: PMC12180227
- DOI: 10.1186/s13058-025-02014-9
Hope for OTHERS (Our Tissue Helping Enhance Research & Science): research results from the University of Pittsburgh rapid autopsy program for breast cancer
Abstract
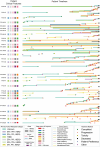
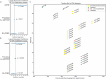
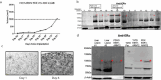
Breast cancer affects 1/8 of women throughout their lifetimes, with over 90% of cancer deaths being caused by metastasis. However, metastasis poses unique challenges to research, as complex changes in the microenvironment in different metastatic sites and difficulty obtaining tissue for study hinder the ability to examine in depth the changes that occur during metastasis. Rapid autopsy programs thus fill a unique need in advancing metastasis research. Here, we describe our protocol and processes for establishing and improving the US-based Hope for OTHERS (Our Tissue Helping Enhance Research and Science) program for organ donation in metastatic breast cancer. As of August 2024, we consented 114 patients and performed 37 autopsies, from which we collected 551 unique metastatic frozen tumor samples, 1244 FFPE blocks, 90 longitudinal liquid biopsy samples and developed 14 patient-derived organoid and 8 patient-derived xenograft models. We report in-depth clinical and histopathological information and discuss extensive new research and novel findings in patient outcomes, metastatic phylogeny, and factors in successful living model development. Our results reveal key logistical and protocol improvements that are uniquely beneficial to certain programs based on identifiable features, such as working closely with patient advocates, methods to rescue RNA quality in cases where tissue quality may degrade due to time delays, as well as guidelines and future expansions of our program.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All research involving human participants was conducted with informed consent obtained according to the ethical guidelines of the University of Pittsburgh Institutional Review Board under STUDY19060376. Consent for publication: All images of individuals on Fig. 3 are from a public website and are of authors who consent to this publication. Competing interests: The authors declare no competing interests.
Figures





Update of
-
Hope for Others: Research Results from the University of Pittsburgh Rapid Autopsy Program for Breast Cancer.bioRxiv [Preprint]. 2025 Mar 12:2024.11.06.621982. doi: 10.1101/2024.11.06.621982. bioRxiv. 2025. Update in: Breast Cancer Res. 2025 Jun 19;27(1):111. doi: 10.1186/s13058-025-02014-9. PMID: 39574596 Free PMC article. Updated. Preprint.
References
-
- Breast Cancer Risk in American Women - NCI. December 17, 2020. Accessed 2 Aug 2024. https://www.cancer.gov/types/breast/risk-fact-sheet
-
- Survival Rates for Breast Cancer. Accessed 1 Feb 2024. https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast...
-
- A Perspective on Cancer Cell Metastasis | Science. Accessed 5 Aug 2023. https://www.science.org/doi/10.1126/science.1203543
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

