Epigenetic editing and epi-drugs: a combination strategy to simultaneously target KDM4 as a novel anticancer approach
- PMID: 40537846
- PMCID: PMC12177974
- DOI: 10.1186/s13148-025-01913-0
Epigenetic editing and epi-drugs: a combination strategy to simultaneously target KDM4 as a novel anticancer approach
Abstract
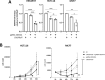
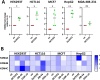
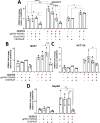
KDM4-A/B/C, preferentially demethylating di- and tri-methylated lysine 9 on histone H3, are overexpressed in cancers and considered interesting therapeutic targets. Consequently, KDM4 inhibitors have been developed to block their enzymatic activity. However, the potential lack of specificity of such small molecules (epi-drugs) may contribute to dose-limiting toxicities. In the pursuit of more specific interventions, epigenetic editing (epi-editing) has emerged as a powerful tool to modulate gene expression by modifying the epigenetic profile of specific genomic locations. The recently developed CRISPRoff (dCas9 fused to DNMT3A/3L and KRAB), guided by sgRNAs, is successfully used for gene repression by introducing methylation of DNA and (indirectly) of histones at the targeted genomic region. We propose that combining epi-editing (here to prevent the expression of KDM4) with epi-drugs (to inhibit the KDM4 protein activity) may represent a novel path for synergistic anticancer effects through simultaneous inhibition of gene expression and protein activity. Upon validating the downregulation of KDM4A in HEK293T cells through epi-editing, we demonstrated its repression in colon, breast and hepatocellular carcinomas which was effective in preventing (breast, MCF7) or inhibiting (colon, HCT116) cancer cell growth. Anticancer effect was also confirmed for these cell lines using the KDM4 inhibitor QC6352. In parallel, our studies demonstrate a previously unnoticed increase in the expression of KDM4-A/B/C genes following the inhibition of protein activity using the pan-KDM4 inhibitors QC6352 and JIB-04. Importantly, this induction of gene expression was fully prevented or even further inhibited by epi-editing. Then, we assessed the efficacy of our dual-targeted silencing approach in cancer cells and demonstrated that the inhibition in cancer cell growth by epi-drug or epigenetic editing could be further improved by combining the treatments. Building upon these findings, we introduce a novel, potentially synergistic, therapeutic strategy that combines epi-drug administration with epi-editing. This innovative approach aims to reduce drug toxicity and the potential development of resistance by preventing drug-induced upregulation of target enzyme expression, thereby further increasing anticancer effects.
Keywords: CRISPR/dCas9; Epi-drugs; Epigenetic editing; JIB-04; KDM4; QC6352.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: The authors declare no competing interests. Consent for publication: Not applicable. Ethics approval: Not applicable.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

