A longitudinal study of the 5xFAD mouse retina delineates Amyloid beta (Aβ)-mediated retinal pathology from age-related changes
- PMID: 40537856
- PMCID: PMC12177965
- DOI: 10.1186/s13195-025-01784-w
A longitudinal study of the 5xFAD mouse retina delineates Amyloid beta (Aβ)-mediated retinal pathology from age-related changes
Abstract
Background: Age-related macular degeneration (AMD) is the commonest cause of irreversible blindness in developed societies. AMD coincides with advanced age to which genetic and lifestyle factors contribute additional risks. High levels of the Alzheimer's-linked Amyloid beta (Aβ) proteins are correlated with aged/AMD retinas. To delineate the role of Aβ in retinopathy from age-related changes, we used transgenic 5xFAD mice in a longitudinal study to recapitulate the aged/AMD Aβ-burden of the human retina.
Methods: Mice were genotyped to exclude the retinal degeneration alleles Pde6brd1, Pde6brd8, Agouti, Tyr and Oca2. Retinas of 5xFAD and wildtype littermates (97 males/females in total) were longitudinally assessed until 15 months using non-invasive retinal scans: multi-focal electroretinography, optokinetic tracking, optical coherence tomography (OCT), colour fundus photography and fluorescein angiography. Mice were killed at 4, 8 and 15 months, and eyes enucleated for analyses by light, confocal and electron microscopy.
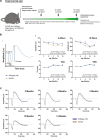
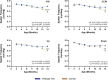
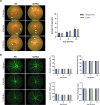
Results: Age-related changes included a gradual decline of retinal activity in all mice. Subretinal/drusen-like deposits increased with age, but, like retinal vessel morphology and vessel integrity, showed no differences between cohorts. Diminished PSD95 levels indicated impaired photoreceptor-bipolar connectivity which correlated with age. Ultrastructural imaging showed increased electron-dense granules and undigested outer segments within retinal pigment epithelial cells with age. 5xFAD pathology included significant weight reduction vs. wildtype/littermates, which were pronounced in females. 8 month old 5xFAD mice had diminished A and B waves, though the age-related decline in wildtype mice abolished these subsequently. Visual acuity/function was also reduced in 14 month 5xFAD eyes. OCT revealed thickened photoreceptor nuclei and inner segments in 8 month 5xFAD retinae. Scrutiny of chorioretinal tissues revealed diminished photoreceptor nuclei in 4 month 5xFAD eyes, though differences were abolished as both cohorts aged. From 8 months onwards, 5xFAD mice possessed fewer bipolar cell nuclei.
Conclusions: Chronic Aβ exposure led to the earlier development of retinopathy-linked features, the identification of which advances our understanding of how Aβ contributes to multifaceted retinopathies. These were distinguishable from wider age-related changes and non-specific influences of retinal degeneration alleles in 5xFAD mice. Longitudinal analyses revealed sex and age-related limitations and important 3Rs considerations for future studies using 5xFAD mice.
Keywords: 5xFAD; Age; Age-related macular degeneration (AMD); Amyloid beta (Aβ); Mouse model; Retina; Retinopathy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate : Studies were overseen by the institutions’ Animal Welfare and Ethical Review Board (AWERB) and were consistent with the Association for Research in Vision and Ophthalmology statement (ARVO) for the Use of Animals in Ophthalmic procedures and the UK Animal (Scientific Procedures) Act of 1986. The study complied with the ARRIVE guidelines with work undertaken under a UK Home Office project licence. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




References
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

