Immune persistence of a single dose of 23-valent pneumococcal polysaccharide vaccine: A 6-year follow-up
- PMID: 40537903
- PMCID: PMC12184158
- DOI: 10.1080/21645515.2025.2517489
Immune persistence of a single dose of 23-valent pneumococcal polysaccharide vaccine: A 6-year follow-up
Erratum in
-
Correction.Hum Vaccin Immunother. 2025 Dec;21(1):2529653. doi: 10.1080/21645515.2025.2529653. Epub 2025 Jul 6. Hum Vaccin Immunother. 2025. PMID: 40619348 Free PMC article. No abstract available.
Abstract
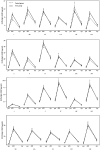
In a previous phase 3 clinical trial, a 23-valent pneumococcal polysaccharide vaccine (PPV23) induced robust immune responses in participants aged 2 years and older. We conducted this study to evaluate the immune persistence of a single dose of PPV23 6 years after vaccination. In this follow-up study, 600 participants aged 2 years and older (referred to the age of vaccination) who had received a single dose of either test vaccine or control vaccine in the previous clinical trial were enrolled in a 3:1 ratio. Blood samples were collected to determine anti-capsular immunoglobulin G (IgG) antibody levels against 23 serotypes with enzyme-linked immunosorbent assay (ELISA). A total of 598 subjects were included for immune persistence analysis. Six years after vaccination, the geometric mean concentrations (GMCs) of IgG antibodies to most serotypes remained higher than prior to vaccination in both groups (1.1-1.8 folds vs.1.1-1.7 folds), although there was a significant decrease compared to 28 days. The results suggested PPV23 could provide protection 6 years after vaccination. Considering the significant decrease of antibody level, the revaccination in high-risk population may be needed.Trial registration: ClinicalTrial.gov identifier: NCT03480763.
Keywords: Immune persistence; pneumococcal polysaccharide vaccine.
Conflict of interest statement
Xinyi Yang is employed by Sinovac Life Sciences Co., Ltd. Taotao Zhu, Zeng Wang, Qingmin Sun, Xing Han are employed by Sinovac Biotech Co., Ltd.
Figures
Similar articles
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Immunogenicity and safety study of 23-valent pneumococcal polysaccharide vaccine revaccination among elderly individuals aged 60-70 years in Shanghai, China.Front Immunol. 2025 Jul 17;16:1623611. doi: 10.3389/fimmu.2025.1623611. eCollection 2025. Front Immunol. 2025. PMID: 40746543 Free PMC article.
-
Effectiveness, immunogenicity and safety of 23-valent pneumococcal polysaccharide vaccine revaccinations in the elderly: a systematic review.BMC Infect Dis. 2016 Nov 25;16(1):711. doi: 10.1186/s12879-016-2040-y. BMC Infect Dis. 2016. PMID: 27887596 Free PMC article.
-
A randomized, blind, parallel controlled phase I clinical trial to evaluate the safety and preliminary immunogenicity of 23-valent pneumococcal polysaccharide vaccine in healthy people aged 2 years and older.Vaccine. 2024 Apr 19;42(11):2858-2866. doi: 10.1016/j.vaccine.2024.03.044. Epub 2024 Mar 21. Vaccine. 2024. PMID: 38519344 Clinical Trial.
-
Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against Pneumococcal Disease in the Elderly: Systematic Review and Meta-Analysis.PLoS One. 2017 Jan 6;12(1):e0169368. doi: 10.1371/journal.pone.0169368. eCollection 2017. PLoS One. 2017. PMID: 28061505 Free PMC article.
References
-
- World Health Organization (WHO) . World health organization position paper on pneumococcal vaccines. Wkly epidemiol Rec. 2012;87:129–7.
-
- Shabil M, Gaidhane S, Ballal S, Kumar S, Bhat M, Sharma S, Kumar MR, Rustagi S, Khatib MN, Rai N, et al. Mortality reduction with 23-valent pneumococcal polysaccharide vaccine: a systematic review and meta-analysis. Pneumonia (Nathan). 2024;16(1):30. doi: 10.1186/s41479-024-00149-5. - DOI - PMC - PubMed
-
- Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(Rr–11):1–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical