Skin ECM Provides a Bio-Derived Platform for Supporting Dermal Renewal and Matrix Synthesis
- PMID: 40537912
- PMCID: PMC12197817
- DOI: 10.4014/jmb.2505.05015
Skin ECM Provides a Bio-Derived Platform for Supporting Dermal Renewal and Matrix Synthesis
Abstract
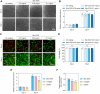
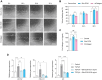
The extracellular matrix (ECM) plays a central role in directing dermal fibroblast behavior and coordinating tissue regeneration through its structural organization and biochemical signaling. In this study, we investigate the composition and regenerative bioactivity of Skin ECM in the context of dermal remodeling as a soluble supplement and surface coating for fibroblast culture. Proteomic profiling demonstrates that Skin ECM preserves the molecular complexity and skin-specific composition of native dermal ECM, including key structural and signaling proteins essential for tissue repair, with over 95% overlap with the human skin proteome, highlighting its strong tissue specificity and biological relevance. Functionally, Skin ECM enhances fibroblast migration during wound healing, upregulates elastin expression, and suppresses transforming growth factor beta 1 (TGF-β1)-induced expression of profibrotic and inflammatory markers, indicating inhibition of fibroblast activation. In vivo subcutaneous implantation confirms high local and systemic biocompatibility without signs of inflammation or toxicity. Collectively, these findings establish Skin ECM as a bioactive, tissue-specific, and immunologically compatible ECM material, offering broad utility in regenerative medicine, three-dimensional (3D) skin model systems, and dermal therapeutics including cosmetic interventions.
Keywords: Skin extracellular matrix; dermal fibroblast; skin regeneration; wound healing.
Conflict of interest statement
Seung-Woo Cho (Chief Executive Officer) and Yoonhee Jin (Chief Technology Officer) are affiliated with Cellartgen. The authors have no financial conflicts of interest to declare.
Figures


Similar articles
-
Microneedle Radiofrequency Induces Extracellular Matrix Remodeling Through Fibroblast Activation: A Histological Study in a Porcine Model.Lasers Surg Med. 2025 Aug;57(6):528-543. doi: 10.1002/lsm.70033. Epub 2025 May 30. Lasers Surg Med. 2025. PMID: 40444432
-
Prediction, screening and characterization of novel bioactive tetrapeptide matrikines for skin rejuvenation.Br J Dermatol. 2024 Jun 20;191(1):92-106. doi: 10.1093/bjd/ljae061. Br J Dermatol. 2024. PMID: 38375775
-
Monopolar Radiofrequency-Induced Fibroblast Stimulation for the Prevention and Improvement of Skin Laxity.Dermatol Surg. 2025 Jul 9;51(9S):S38-S43. doi: 10.1097/DSS.0000000000004714. Dermatol Surg. 2025. PMID: 40864850 Review.
-
T16 modulated extracellular matrix remodeling in fibroblasts via paracrine activation of TGF-β1 through M2 macrophage polarization.Mol Biol Rep. 2025 Jun 26;52(1):642. doi: 10.1007/s11033-025-10733-7. Mol Biol Rep. 2025. PMID: 40569521
-
Targeting Dermal Fibroblast Senescence: From Cellular Plasticity to Anti-Aging Therapies.Biomedicines. 2025 Aug 7;13(8):1927. doi: 10.3390/biomedicines13081927. Biomedicines. 2025. PMID: 40868181 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

