Indirect comparative efficacy and safety of tirzepatide 10 and 15 mg versus semaglutide 2.4 mg for the management of obesity and overweight in patients with type 2 diabetes
- PMID: 40537987
- PMCID: PMC12326929
- DOI: 10.1111/dom.16508
Indirect comparative efficacy and safety of tirzepatide 10 and 15 mg versus semaglutide 2.4 mg for the management of obesity and overweight in patients with type 2 diabetes
Abstract
Aims: This indirect treatment comparison (ITC) compared the efficacy and safety of tirzepatide with semaglutide for managing obesity or overweight in participants with type 2 diabetes (T2D), informed by the pivotal trials SURMOUNT-2 and STEP 2.
Materials and methods: Participants had body mass index (BMI) ≥ 27 kg/m2, with ≥1 unsuccessful prior dietary weight reduction effort and glycated haemoglobin (HbA1c) 7%-10% on stable therapy. A heterogeneity assessment confirmed that study and patient baseline characteristics were similar. Bucher ITCs compared tirzepatide 10 and 15 mg once-weekly (QW) to semaglutide 2.4 mg QW via placebo, all adjunct to a reduced-calorie diet and increased physical activity.
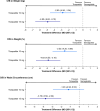
Results: Tirzepatide 10 and 15 mg were associated with statistically significant greater reductions in weight, BMI and HbA1c versus semaglutide. Tirzepatide 15 mg was associated with statistically significant greater odds versus semaglutide of ≥5% and ≥15% weight reduction and statistically significant improvements in several cardiometabolic risk factors, including waist circumference, fasting plasma glucose and triglycerides. Both tirzepatide doses showed non-significant trends of greater improvements in high-density lipoprotein, low-density lipoprotein, systolic blood pressure and diastolic blood pressure versus semaglutide as well as a generally comparable safety profile to semaglutide.
Conclusions: In this ITC versus semaglutide 2.4 mg, tirzepatide 10 and 15 mg were associated with statistically significant greater weight, BMI and HbA1c reduction and tirzepatide 15 mg with statistically significant improvements in multiple cardiometabolic risk factors crucial in managing obesity or overweight among patients with T2D. Both tirzepatide doses also had a generally similar safety profile to semaglutide.
Plain language summary: What is the context and purpose of this research study? Excess weight and type 2 diabetes (T2D) are strongly connected, where most patients with T2D have obesity or overweight. Weight management is crucial for improving T2D outcomes and preventing its progression. Weight management comprises behavioural interventions, psychological support, dietary changes and physical activity programmes. Medications may also be prescribed or surgical options may also be considered. Two such medications for weight management are tirzepatide (up to 15 mg) and semaglutide (up to 2.4 mg), which are injected subcutaneously once per week to help control appetite by prolonging patients' feeling of fullness. These medications are also used at different doses to treat T2D. Because there were no clinical trials directly comparing tirzepatide and semaglutide, particularly in patients with both T2D and either obesity or overweight, this study aimed to indirectly compare the effectiveness and safety of tirzepatide and semaglutide for weight management in patients with overweight or obesity and T2D. What was done? We indirectly compared the efficacy and safety of two doses of tirzepatide (10 and 15 mg per week) versus semaglutide 2.4 mg per week for weight management in adults with both T2D and either obesity or overweight. We used data from two large clinical trials, SURMOUNT-2 and STEP 2, which tested tirzepatide and semaglutide, respectively, against a placebo, all adjunct to diet and exercise. An indirect treatment comparison of tirzepatide and semaglutide was then possible via the placebo arm acting as the common comparator. The similarity of study design and patient populations in the two trials was evaluated and found to be sufficiently close to allow meaningful comparisons. Appropriate statistical methodology was used to facilitate comparisons of the two trials. What were the main results? Compared to semaglutide 2.4 mg, the higher dose of tirzepatide (15 mg) was associated with a statistically significant improvement in several outcomes such as weight reduction, glycaemic outcomes and triglycerides, while the lower dose of tirzepatide (10 mg) was associated with some statistically significant improvements (e.g., weight reduction and HbA1c) and had otherwise comparable outcomes to semaglutide. However, both doses of tirzepatide were associated with statistically significant greater reductions in glycated haemoglobin A1c (HbA1c) compared to semaglutide, which is a key target of T2D treatment. Both doses of tirzepatide had a generally similar safety profile compared to semaglutide. What is the originality and relevance of this study? Currently, there are no clinical trials that compare tirzepatide and semaglutide directly for the management of obesity and overweight in patients with T2D. Previous studies have compared tirzepatide and semaglutide results from different clinical trials for weight management in patients without T2D, not specifically focusing on patients with T2D. This is the first study to indirectly compare tirzepatide and semaglutide for weight management in people with T2D who also have obesity or overweight. The findings of this study suggest that higher doses of tirzepatide may be more effective than semaglutide for weight reduction and improving other health-related outcomes in these patients.
Keywords: GIP; GLP‐1; type 2 diabetes; weight management.
© 2025 Eli Lilly and The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
AC: Honoraria from AstraZeneca, Boehringer Ingelheim, Lilly, Novo Nordisk; Member of the DMC of Boehringer Ingelheim clinical trials–SYNCRONIZE program; EJ, SZR, GKD, MB, LF, HS: Employee and shareholder of Eli Lilly and Company; TC, LJC: Employees of Costello Medical, which received payment from Eli Lilly and Company for analytical services for this study; JFB: Received honoraria from Abbvie, Amgen, Astellas, AstraZeneca, Bayer, BeiGene, Gilead, GSK, IQVIA, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Takeda.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

