Rearing Behavior as Indicator of Spatial Novelty and Memory in Developing Rats
- PMID: 40538152
- PMCID: PMC12179583
- DOI: 10.1111/ejn.70162
Rearing Behavior as Indicator of Spatial Novelty and Memory in Developing Rats
Abstract
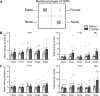
Among the various forms of exploration, rearing-where rodents stand on their hind legs-reflects the animal's processing of spatial information and response to environmental novelty. Here, we investigated the developmental trajectory of rearing in response to spatial novelty in a standard object-place recognition (OPR) task, with the OPR retrieval phase allowing for a direct comparison of measures of rearing, object exploration, and locomotion as indicators of spatial novelty and memory. Groups of male rats were tested on postnatal day (PD) 25, PD31, PD38, PD48, and at adulthood (PD84). The OPR task comprised a 5-min encoding phase with the rat exposed to an arena with two identical objects and, 3 h later, a 5-min retrieval phase in the same arena with one object being displaced to another arena zone. Rearing increased in response to spatial novelty (i.e., the displaced object) at retrieval relative to encoding, with this increase occurring first on PD31, and thus later than preferential object exploration-based responses emerging already on PD25. Importantly, zone-specific analyses during retrieval revealed an increase in rearing events in the (now empty) zone where the displaced object is used to be at encoding. This increase was only observed in adult rats (PD84) and likely indicates the presence of specific object-place associations in memory. These findings evidence rearing as behavior covering aspects of spatial novelty complementary to those of object exploration, thereby enabling a more comprehensive characterization of the emergence of spatial episodic memory during early life.
Keywords: developmental trajectory; exploratory behavior; object–place recognition; spatial episodic memory; spatial learning.
© 2025 The Author(s). European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Akers, K. G. , and Hamilton D. A.. 2007. “Comparison of Developmental Trajectories for Place and Cued Navigation in the Morris Water Task.” Developmental Psychobiology 49: 553–564. - PubMed
-
- Barker, G. R. , Bird F., Alexander V., and Warburton E. C.. 2007. “Recognition Memory for Objects, Place, and Temporal Order: A Disconnection Analysis of the Role of the Medial Prefrontal Cortex and Perirhinal Cortex.” Journal of Neuroscience 27, no. 11: 2948–2957. 10.1523/JNEUROSCI.5289-06.2007. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

