Label-Free Single-Molecule Immunoassay
- PMID: 40538199
- PMCID: PMC12376538
- DOI: 10.1002/advs.202505207
Label-Free Single-Molecule Immunoassay
Abstract
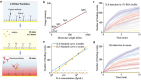
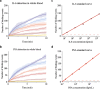
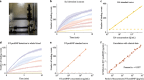
Single-molecule immunoassay is a reliable technique for the detection and quantification of low-abundance blood biomarkers, which are essential for early disease diagnosis and biomedical research. However, current single-molecule methods predominantly rely on endpoint detection and necessitate signal amplification via labeling, which brings a variety of unwanted effects, like matrix effect and autofluorescence interference. This study introduces a real-time mass imaging-based label-free single-molecule immunoassay (LFSMiA). Featuring plasmonic scattering microscopy-based mass imaging, a 2-step sandwich assay format enables background reduction, minimization of matrix effect by dynamic tracking of single binding events, and fully leveraging real-time data for improved measurement precision through a Bayesian Gaussian process model, the LFSMiA enables ultra-sensitive and direct protein detection at the single-molecule level in neat blood sample matrices. LFSMiA measurement is demonstrated for interleukin-6 and prostate-specific antigen in buffer, undiluted serum, and whole blood with sub-femtomolar detection limits and eight logs of dynamic ranges. Moreover, comparable performance is achieved with an inexpensive miniaturized setup. To show its translational potential to clinical settings and point-of-care diagnostics, N-terminal pro-B-type natriuretic peptide is examined in patient whole blood samples using the LFSMiA and results in a strong linear correlation (r > 0.99) with standard clinical lab results.
Keywords: digital immunoassay; label‐free; plasmonic scattering microscopy; single‐molecule; whole blood.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare the following competing financial interest(s): S.W. is a member of the technology advisory board of Biosensing Instrument Inc. A US patent application (18/956,466) has been filed by the Arizona Board of Regents on behalf of Arizona State University based on an early draft of this article. The inventors are S.W., X.Z, and S.Z.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
