Organization and development of bilateral somatosensory feedback projections in mice
- PMID: 40538433
- PMCID: PMC12177178
- DOI: 10.1016/j.isci.2025.112725
Organization and development of bilateral somatosensory feedback projections in mice
Abstract
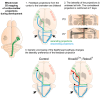
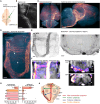
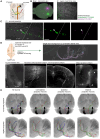
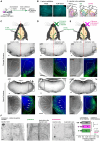
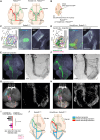
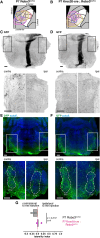
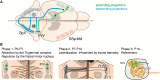
Sensory information travels from the periphery to the cortex across relays. Each of these stations receives feedback projections which gate and tune the transmission of feedforward information. We used whole brain imaging combined with viral strategies and single axon tracings to reveal the existence of a bilateral feedback projection from the somatosensory cortex to brainstem relays processing whisker information. Initially, after birth in mice, this feedback loop projects equally to both sides of the brainstem. Then, the projection strengthens preferentially toward the contralateral side while maintaining its ipsilateral component. We found that manipulating the laterality of the feedforward pathway from the brainstem to the opposite cortex altered the laterality preference of the descending feedback projections. This suggests a dynamic interplay between ascending and descending pathways in shaping sensory processing. This study highlights the presence of bilateral integration within the somatosensory system's feedback projections.
Keywords: Biological sciences; Natural sciences; Neuroanatomy; Neuroscience; Systems neuroscience.
© 2025 The Author(s).
Conflict of interest statement
Authors declare that they have no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

