Melatonin Improves Quality of Life, Oxidative Stress, and Cardiovascular Function in Pulmonary Arterial Hypertension
- PMID: 40538465
- PMCID: PMC12177658
- DOI: 10.1002/pul2.70109
Melatonin Improves Quality of Life, Oxidative Stress, and Cardiovascular Function in Pulmonary Arterial Hypertension
Abstract
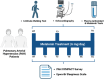
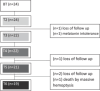
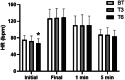
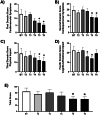
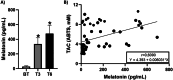
Pulmonary arterial hypertension (PAH) Group 1 from the World Health Organization (WHO) is a rare, severe chronic, and progressive condition. Patients with PAH have increased oxidative stress (OS) and diminished antioxidant capacity. Melatonin is a potent antioxidant hormone with reported benefits in PAH animal models. We aimed to evaluate the functional, hemodynamic, and antioxidant response to a 6-month melatonin therapy in PAH Group 1 patients. Clinical evaluation was done at baseline (BT), as well as at 3 (T3) and 6 (T6) months of melatonin treatment in stable PAH Group 1 (WHO) patients. The principal endpoint was change in walking distance (WD) in the 6-min walking test (6MWT). Secondary endpoints were functional class (FC), quality of life (QoL), performance of right ventricle (RV), and plasma antioxidant capacity. This study included 19 patients. They were mainly women in WHO FC II and III. A significant improvement was noticed in perception of dyspnea, palpitations, and fatigue in concordance with the QoL improvement in the physical domain after 6 months of melatonin. This was represented by a significant fall in the total score of the PAH-SYMPACT questionnaire. In addition, the baseline heart rate was lower at the T6 follow-up. No significant changes were seen in the echocardiographic variables. However, the biochemical analysis showed significative increases in plasma total antioxidant (2.94 ± 0.13 vs. 8.41 ± 0.19) and ferric reducing (191 ± 12 vs. 256 ± 17) capacities. Overall, oral melatonin treatment improved the plasma antioxidant capacity and the QoL in this pilot study.
Keywords: FRAP; antioxidant capacity; cardiac remodeling; life quality; pulmonary hypertension; right ventricle dysfunction.
© 2025 The Author(s). Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
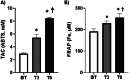
References
-
- Humbert M., Kovacs G., Hoeper M. M., et al., “2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension,” European Heart Journal 43, no. 38 (October 2022): 3618–3731. - PubMed
-
- Humbert M., Sitbon O., Chaouat A., et al., “Survival in Patients With Idiopathic, Familial, and Anorexigen‐Associated Pulmonary Arterial Hypertension in the Modern Management Era,” Circulation 122, no. 2 (July 2010): 156–163. - PubMed
-
- Beshay S., Sahay S., and Humbert M., “Evaluation and Management of Pulmonary Arterial Hypertension,” Respiratory Medicine 171 (September 2020): 106099. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

