Longitudinal computed tomography-based delta-radiomics of visceral adipose tissue predicts infliximab secondary loss of response in Crohn's disease patients
- PMID: 40538512
- PMCID: PMC12175844
- DOI: 10.3748/wjg.v31.i21.105895
Longitudinal computed tomography-based delta-radiomics of visceral adipose tissue predicts infliximab secondary loss of response in Crohn's disease patients
Abstract
Background: Visceral adipose tissue (VAT) plays a role in the pathogenesis of Crohn's disease (CD) and is associated with treatment outcomes following infliximab (IFX) therapy. We developed and validated the first delta-radiomics model to quantify VAT heterogeneity as a predictive biomarker for IFX response in patients with CD.
Aim: To develop a longitudinal computed tomography (CT)-based delta-radiomics model of VAT for predicting secondary loss of response (SLR) in patients with CD.
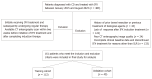
Methods: This retrospective study included 161 patients with CD who achieved clinical remission following IFX induction therapy between 2015 and 2023. All patients underwent CT enterography before IFX initiation and after completing induction therapy. VAT volume was delineated by two radiologists in consensus. Radiomics features were extracted from pre-treatment and post-induction CT images, and delta-radiomics features were calculated as follows: Delta features = Feature-post - Feature-pre. A radiomics model was constructed using logistic regression. Model performance was assessed using discrimination, calibration, and decision curve analyses.
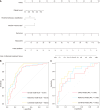
Results: Nine significant delta-radiomics features were used to develop the delta-radiomics model, yielding an area under the receiver operating characteristic curve (AUC) of 0.816 (95%CI: 0.737-0.896) in the training cohort and 0.750 (95%CI: 0.605-0.895) in the validation cohort. Multivariable logistic regression identified platelet count, Montreal behavior classification, and the VAT/subcutaneous adipose tissue volume ratio prior to treatment as independent risk factors for SLR. The combined model integrating clinical predictors and delta-radiomics features achieved superior predictive performance, with an AUC of 0.853 (95%CI: 0.786-0.921) in the training cohort and 0.812 (95%CI: 0.677-0.948) in the validation cohort.
Conclusion: We developed a predictive model based on longitudinal changes in VAT, demonstrating significant potential for identifying patients with CD at high risk of SLR to IFX therapy.
Keywords: Computed tomography enterography; Crohn’s disease; Delta-radiomics; Infliximab; Secondary loss of response.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures


Similar articles
-
Psoas muscle CT radiomics-based machine learning models to predict response to infliximab in patients with Crohn's disease.Ann Med. 2025 Dec;57(1):2527954. doi: 10.1080/07853890.2025.2527954. Epub 2025 Jul 5. Ann Med. 2025. PMID: 40616584 Free PMC article.
-
Correlation between radiomic features of Crohn's disease and secondary loss of response to infliximab.World J Gastroenterol. 2025 Jul 21;31(27):109459. doi: 10.3748/wjg.v31.i27.109459. World J Gastroenterol. 2025. PMID: 40741098 Free PMC article.
-
CT-based delta-radiomics signature of visceral adipose tissue for prediction of disease progression in ileal stricturing Crohn's disease.Jpn J Radiol. 2025 Aug;43(8):1335-1346. doi: 10.1007/s11604-025-01779-5. Epub 2025 Apr 11. Jpn J Radiol. 2025. PMID: 40214913
-
Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease.Cochrane Database Syst Rev. 2018 May 12;5(5):CD012540. doi: 10.1002/14651858.CD012540.pub2. Cochrane Database Syst Rev. 2018. PMID: 29756637 Free PMC article.
-
Stem cell transplantation for induction of remission in medically refractory Crohn's disease.Cochrane Database Syst Rev. 2022 May 13;5(5):CD013070. doi: 10.1002/14651858.CD013070.pub2. Cochrane Database Syst Rev. 2022. PMID: 35556242 Free PMC article.
References
-
- Dolinger M, Torres J, Vermeire S. Crohn's disease. Lancet. 2024;403:1177–1191. - PubMed
-
- Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531–1555. - PubMed
-
- Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn's disease. Aliment Pharmacol Ther. 2018;48:394–409. - PubMed
-
- Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

