Recent advances and challenges in colorectal cancer: From molecular research to treatment
- PMID: 40538516
- PMCID: PMC12175868
- DOI: 10.3748/wjg.v31.i21.106964
Recent advances and challenges in colorectal cancer: From molecular research to treatment
Abstract
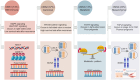
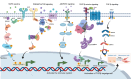
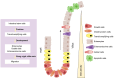
Colorectal cancer (CRC) ranks among the top causes of cancer-related fatalities globally. Recent progress in genomics, proteomics, and bioinformatics has greatly improved our comprehension of the molecular underpinnings of CRC, paving the way for targeted therapies and immunotherapies. Nonetheless, obstacles such as tumor heterogeneity and drug resistance persist, hindering advancements in treatment efficacy. In this context, the integration of artificial intelligence (AI) and organoid technology presents promising new avenues. AI can analyze genetic and clinical data to forecast disease risk, prognosis, and treatment responses, thereby expediting drug development and tailoring treatment plans. Organoids replicate the genetic traits and biological behaviors of tumors, acting as platforms for drug testing and the formulation of personalized treatment approaches. Despite notable strides in CRC research and treatment - from genetic insights to therapeutic innovations - numerous challenges endure, including the intricate tumor microenvironment, tumor heterogeneity, adverse effects of immunotherapies, issues related to AI data quality and privacy, and the need for standardization in organoid culture. Future initiatives should concentrate on clarifying the pathogenesis of CRC, refining AI algorithms and organoid models, and creating more effective therapeutic strategies to alleviate the global impact of CRC.
Keywords: Artificial intelligence; Colorectal cancer; Molecular; Organoid; Treatment.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures




Similar articles
-
The Use of AI for Phenotype-Genotype Mapping.Methods Mol Biol. 2025;2952:369-410. doi: 10.1007/978-1-0716-4690-8_21. Methods Mol Biol. 2025. PMID: 40553344
-
Integrating Genetic Insights and Artificial Intelligence for Enhanced Oral and Maxillofacial Cancer Care.Methods Mol Biol. 2025;2952:107-124. doi: 10.1007/978-1-0716-4690-8_7. Methods Mol Biol. 2025. PMID: 40553330 Review.
-
Implications of Artificial Intelligence for Colorectal Cancer in Young Populations.J Surg Oncol. 2025 Jun;131(7):1368-1372. doi: 10.1002/jso.28036. Epub 2024 Dec 9. J Surg Oncol. 2025. PMID: 39648729 Review.
-
Artificial Intelligence Can Predict Personalized Immunotherapy Outcomes in Cancer.Cancer Immunol Res. 2025 Jul 2;13(7):964-977. doi: 10.1158/2326-6066.CIR-24-1270. Cancer Immunol Res. 2025. PMID: 40499001 Review.
-
AI-Driven Antimicrobial Peptide Discovery: Mining and Generation.Acc Chem Res. 2025 Jun 17;58(12):1831-1846. doi: 10.1021/acs.accounts.0c00594. Epub 2025 Jun 3. Acc Chem Res. 2025. PMID: 40459283 Free PMC article. Review.
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Filho AM, Laversanne M, Ferlay J, Colombet M, Piñeros M, Znaor A, Parkin DM, Soerjomataram I, Bray F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int J Cancer. 2025;156:1336–1346. - PubMed
-
- Burnett-Hartman AN, Murphy CC, Lee JK. Novel, Emerging Risk Factors for Colorectal Cancer Remain Understudied. Gastroenterology. 2022;163:574–576. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

