Tralokinumab as a Therapeutic Alternative for Dupilumab-associated Arthralgia in Atopic Dermatitis: A Multi-center Case Series
- PMID: 40538523
- PMCID: PMC12175839
Tralokinumab as a Therapeutic Alternative for Dupilumab-associated Arthralgia in Atopic Dermatitis: A Multi-center Case Series
Abstract
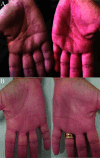
Atopic dermatitis (AD) is a chronic inflammatory skin condition that often requires systemic treatment to achieve optimal clinical outcomes. The clinical and immunological heterogeneity of AD necessitates the use of various therapies to maximize efficacy while minimizing adverse events (AEs). Dupilumab, the first biologic agent approved by the United States Food and Drug Administration (FDA) for moderate-to-severe AD, targets interleukin-4 (IL-4) and interleukin-13 (IL-13) signaling pathways. Although effective, some patients experience dupilumab-associated musculoskeletal AEs, such as arthralgia, arthritis, or enthesitis, which may lead to discontinuation of treatment. Recent studies suggest that IL-4 inhibition disrupts T-cell populations, promoting a skewed T-helper 17 (Th17)-dominant immune response that may contribute to arthralgia. Switching to alternative therapies, such as tralokinumab-an IL-13-specific inhibitor-has shown promise in alleviating these AEs while maintaining control of AD signs and symptoms. Case reports indicate that patients with dupilumab-associated arthralgia have improved after switching to tralokinumab, suggesting the potential of tralokinumab as a safer alternative for these individuals. We present a series of 15 AD patients treated with tralokinumab following discontinuation of dupilumab due to arthralgia. All 15 patients achieved clear or nearly clear skin and demonstrated reductions in AD signs and symptoms as measured by Investigator's Global Assessment (IGA), body surface area of involvement (BSA), and/or patient reported measures of pruritus. Importantly, all patients experienced resolution of arthralgia without recurrence while on tralokinumab. These findings support the use of tralokinumab as an effective and safe alternative therapy for patients with dupilumab-induced arthralgia.
Keywords: Joint pain; adverse event; biologic therapy; eczema; prurigo nodularis.
Copyright © 2025. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: Dr. Shahriari has served as a consultant for AbbVie, Apogee, Arcutis, Bristol Myers Squibb, Dermavant, Galderma, Incyte, Janssen, Leo Pharma, Lilly USA, Novartis, Novan, Ortho Dermatologics, Sanofi-Genzyme, Regeneron, and UCB; a speaker for Abbvie, Arcutis, Bristol Myers Squibb, Lilly USA, Janssen, Dermavant, Leo Pharma (former), Sanofi-Genzyme, Regeneron, and UCB; and an investigator at AbbVie, CorEvitas Psoriasis Registry, CorEvitas Atopic Dermatitis Registry, Dermira, Lilly USA, Cara, Dermavant, Novartis, Union, and Mindera. Dr. Cameron has served as a consultant for Abbvie, Apogee, Arcutis, Bristol Myers Squibb, CorEvitas Atopic Dermatitis Registry, CorEvitas Alopecia Areata Registry, Dermavant Sciences, Eli Lilly, Evelo, Galderma, Incyte, Journey Medical, Leo Pharma, Regeneron, Sanofi, Sun, Union Therapeutics, Verrica; a promotional speaker for Abbvie, Amgen, Bristol Myers Squibb, Dermavant Sciences, Eli Lilly, Incyte, Journey Medical, Leo Pharma, Pfizer, Regeneron, Sanofi, Ortho Pharmaceutical, Verrica; an investigator for Abbvie, Alumis, Apogee, Incyte, Eli Lilly, Novartis, Sanofi, Sun Pharma. Dr. Payette has served as consultant for Abbvie, Amgen, Arcutis, Boehringer Ingelheim, Bristol Meyers Squibb, Castle Biosciences, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, Leo Pharmaceuticals, Novartis, ONVIV, Ortho-Dermatologics, Pfizer, Sanofi-Genzyme, Sun Pharmaceuticals, Regeneron, Takeda, and UCB; a speaker for Abbvie, Amgen, Arcutis, Bristol Meyers Squibb, Eli Lilly, Galderma, Incyte, Janssen, Novartis, Pfizer, Sanofi-Genzyme, Sun Pharmaceuticals, Regeneron, and UCB; and an investigator for Abbvie, Cara, CorEvitas Atopic Dermatitis Registry, CorEvitas Psoriasis Registry, Dermavant, Dermira, Eli-Lilly, Incyte, Mindera, and Novartis. Dr. Dasilva has served as a speaker or consultant for AbbVie, Arcutis, Dermavant, Eli Lilly, Galderma, Janssen, LEO pharma, Pfizer, Sanofi and Regeneron, UCB, Verrica. Dr. Damiani has served as a consultant for Almirall, Galderma, Funziona, and a speaker for Almirall, Galderma, Funziona, and Sanofi. Dr. Herman has served as a consultant, speaker, or investigator for AbbVie, Bristol Myers Squibb, LEO Pharma, Lilly, Regeneron, and Sanofi. Dr. Issa has served as a speaker, consultant, advisor, or investigator for Abbvie, Almirall, Apogee, Bristol Myers Squibb, Castle Biosciences, Dermavant Sciences, DermTech, Galderma, Incyte, Janssen, Journey, LEO Pharma, Lilly, Novartis, Ortho Dermatologics, Pfizer, Primus, Regeneron, Sanofi, SUN Pharmaceuticals Industry, Topix, UCB, Verrica Pharmaceuticals. Dr. Rodriguez has served as a consultant, research investigator and/or speaker for Abbvie, Arcutis, Almirall, Bristol Myers Squibb, Dermavant, Galderma, Incyte, Janssen, Johnson & Johnson, Leo Pharma, Lilly, Mindera, Novan, Novartis, Sanofi, Sun, and UCB. Dr. Del Rosso has served as a consultant, research investigator, and/or speaker for Abbvie, Amgen, Arcutis, Almirall, Bausch Health, Botanix, Bristol Myers Squibb, Cara, Dermavant, Evommune, Ferndale, Galderma, Incyte, Journey Leo Pharma, Lilly USA, Mindera, Moonlake, Nektar, Novan, Novartis, Primus, Regeneron, Sanofi-Genzyme, Sun Pharma, UCB and Vyne. Dr. Cohen serves on a data and safety monitoring board for Advarra and has served as a consultant for Novartis, Takeda, and GSK. Dr. Bunick has served as an investigator for AbbVie, Almirall, Apogee, Daiichi Sankyo, LEO Pharma, Ortho Dermatologics, Sun Pharma, Timber, and Palvella; a consultant for AbbVie, Almirall, Apogee, Arcutis, Connect BioPharma, Eli Lilly, EPI Health/Novan, Incyte, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Sanofi-Regeneron, Takeda, and UCB; and a speaker for and received honoraria from Allergan, Almirall, LEO Pharma, and UCB.
Figures
References
-
- de Wijs LEM, van der Waa JD, de Jong PHP et al. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol. 2020;45(2):262–263. - PubMed
-
- Chrétien B, Dolladille C, Alexandre J et al. Dupilumab-associated arthralgia: an observational retrospective study in VigiBase®. Br J Dermatol. 2021;185(2):464–465. - PubMed
-
- Napolitano M, Ruggiero A, Patruno C. Dupilumab-associated inflammatory arthritis: a literature review. Clin Exp Dermatol. 2024;49(4):307–312. - PubMed
-
- Woodbury MJ, Smith JS, Merola JF. Dupilumab-associated arthritis: A dermatology-rheumatology perspective. Am J Clin Dermatol. 2023;24(6):859–864. - PubMed
-
- Bridgewood C, Sharif K, Freeston J et al. Regulation of entheseal IL-23 expression by IL-4 and IL-13 as an explanation for arthropathy development under dupilumab therapy. Rheumatology (Oxford). 2021;60(5):2461–2466. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
