Real-world treatment persistence and predictive factors for discontinuation of antifibrotic therapies in patients with idiopathic pulmonary fibrosis: a post-hoc analysis of two multicenter observational cohort studies in Poland
- PMID: 40538535
- PMCID: PMC12177363
- DOI: 10.3389/fphar.2025.1586197
Real-world treatment persistence and predictive factors for discontinuation of antifibrotic therapies in patients with idiopathic pulmonary fibrosis: a post-hoc analysis of two multicenter observational cohort studies in Poland
Abstract
Background: Persistence with antifibrotic medications in patients with idiopathic pulmonary fibrosis (IPF) is crucial for long-term outcomes. However, real-world data regarding treatment persistence patterns in IPF are scarce.
Methods: We conducted a post hoc analysis of two retrospective, real-world, multicenter observational studies (PolExPIR and PolExNIB) that collected clinical data on Polish patients with IPF managed at specialized centers between January 2017 and October 2021. We compared clinical variables between groups of patients who continued and discontinued antifibrotics and evaluated predictive factors for treatment discontinuation.
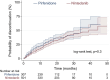
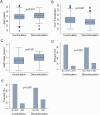
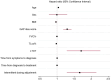
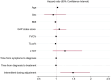
Results: Overall, 808 patients were included in the analysis. Of these, 278 subjects (34.4%) discontinued therapy over a median follow-up of 16 (8-24) months. The proportion of patients discontinuing therapy was comparable between pirfenidone and nintedanib (37.5% vs. 32.5% respectively; p = 0.15). Additionally, no statistical difference was observed between antifibrotic agents in the distribution of time until treatment discontinuation (log-rank test, p = 0.3). Predictive factors associated with the probability of treatment discontinuation included age (hazard ratio [HR] 1.04; 95% confidence interval [CI] 1.02-1.05), body mass index (BMI, HR 0.97; 95% CI 0.94-0.99), transfer factor of the lung for carbon monoxide (TLco)% predicted (HR 0.98, 95% CI 0.97-0.99), Gender-Age-Physiology (GAP) index score (HR 1.3, 95% CI 1.18-1.42), use of long-term oxygen therapy (LTOT) (HR = 1.7, 95% CI 1.28-2.27) and intermittent dosing adjustment (HR 1.66, 95% CI 1.29-2.15).
Conclusion: In this large population-based cohort of patients with IPF, around one-third discontinued antifibrotics during a study follow-up with no difference in the rates and time to discontinuation between pirfenidone and nintedanib. Clinical predictive factors including age, BMI, TLco% predicted, GAP index score, use of LTOT and intermittent dosing adjustment were associated with the risk of treatment discontinuation.
Keywords: IPF; antifibrotic therapy; idiopathic pulmonary fibrosis; nintedanib; pirfenidone; predictive factors; treatment discontinuation; treatment persistence.
Copyright © 2025 Majewski, Górska, Lewandowska, Martusewicz-Boros, Sobiecka and Piotrowski.
Conflict of interest statement
SM, KG, KL, MM-B, MS, and WP declares receiving grants and personal fees from Boehringer Ingelheim and Roche outside of submitted work.
Figures
References
-
- Antoniou K., Markopoulou K., Tzouvelekis A., Trachalaki A., Vasarmidi E., Organtzis J., et al. (2020). Efficacy and safety of nintedanib in a Greek multicentre idiopathic pulmonary fibrosis registry: a retrospective, observational, cohort study. ERJ Open Res. 6 (1), 00172-2019. 10.1183/23120541.00172-2019 - DOI - PMC - PubMed
-
- Baumgartner K. B., Samet J. M., Coultas D. B., Stidley C. A., Hunt W. C., Colby T. V., et al. (2000). Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Collab. Centers. Am. J. Epidemiol. 152 (4), 307–315. 10.1093/aje/152.4.307 - DOI - PubMed
-
- Bhomra P. S., Burge G. A., Turner A. M., Burge P. S., Walters G. I. (2018). P158 Which factors predict early discontinuation of antifibrotics in idiopathic pulmonary fibrosis? Thorax 73 (Suppl. 4), A188. 10.1136/thorax-2018-212555.316 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

