1,8-Cineole inhibits platelet-leukocyte aggregate formation by reducing P-selectin expression
- PMID: 40538536
- PMCID: PMC12176828
- DOI: 10.3389/fphar.2025.1546157
1,8-Cineole inhibits platelet-leukocyte aggregate formation by reducing P-selectin expression
Erratum in
-
Correction: 1,8-Cineole inhibits platelet-leukocyte aggregate formation by reducing P-selectin expression.Front Pharmacol. 2025 Jul 10;16:1656591. doi: 10.3389/fphar.2025.1656591. eCollection 2025. Front Pharmacol. 2025. PMID: 40709091 Free PMC article.
Abstract
Introduction: Platelets, traditionally recognized for their role in hemostasis, have increasingly been implicated in cancer progression, including head and neck squamous cell carcinoma (HNSCC). Beyond releasing growth factors and chemokines, platelets modulate leukocyte-mediated proinflammatory responses and effector functions through direct or indirect contact. These processes promote tumor cell proliferation, survival, epithelial to mesenchymal transition (EMT) and extravasation. Consequently, targeting platelet-leukocyte aggregate (PLA) formation represents a promising pharmacological strategy to interfere with platelet-mediated pro-tumorigenic effects. 1,8-cineole, a plant-derived metabolite found in several botanical sources, has shown potent anti-platelet effects through modulation of the adenosine A2A receptor signaling. However, its influence on PLA formation has not been investigated.
Methods: In this study, we analyzed platelet activation and PLA formation in HNSCC patients compared to healthy donors. A co-culture system combined with blocking antibodies was employed to elucidate the mechanisms of PLA formation. Moreover, the pharmacological effects of 1,8-cineole were compared with those of conventional anti-platelet drugs.
Results: The results revealed elevated P-selectin expression and enhanced PLA formation in HNSCC patients. PLA formation was predominantly mediated through P-selectin-PSGL-1 interactions. Ex vivo studies demonstrated that 1,8-cineole significantly reduced PLA formation by inhibiting P-selectin expression on platelets. Notably, traditional anti-platelet agents did not significantly inhibit PLA formation, despite effectively reducing platelet aggregation.
Discussion: These findings identify a pharmacological effect of 1,8-cineole in disrupting platelet-leukocyte interactions via suppression of the P-selectin-PSGL-1 axis. This suggests that 1,8-cineole offers potential pharmacological benefits in mitigating platelet-mediated inflammation and tumor progression.
Keywords: 1,8-cineole; anti-platelet drugs; head and neck squamous cell carcinoma; platelet-leukocyte aggregates; platelets.
Copyright © 2025 Petry, Mai, Shoykhet, Bashiri Dezfouli and Wollenberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

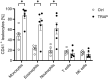


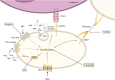
Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Pathogen-reduced platelets for the prevention of bleeding.Cochrane Database Syst Rev. 2013 Mar 28;(3):CD009072. doi: 10.1002/14651858.CD009072.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jul 30;7:CD009072. doi: 10.1002/14651858.CD009072.pub3. PMID: 23543569 Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
References
LinkOut - more resources
Full Text Sources

