Aqueous extracts of Portulaca oleracea L. alleviate atopic dermatitis by restoring skin barrier function
- PMID: 40538548
- PMCID: PMC12176770
- DOI: 10.3389/fphar.2025.1591394
Aqueous extracts of Portulaca oleracea L. alleviate atopic dermatitis by restoring skin barrier function
Abstract
Background: Portulaca oleracea L. (PO) is an edible plant with a long medicinal history in traditional Chinese medicine for various inflammatory diseases, including skin disorders such as atopic dermatitis (AD). However, the anti-inflammatory effects and AD-alleviating mechanisms of PO remain unclear.
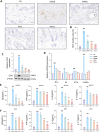
Methods: PO aqueous extract was prepared from a water-soluble portion and then mixed with carbomer to obtain a hydrogel, which provided stable drug permeation and absorption in mouse skin. Amantadine acetate was identified as an abundant ingredient and further predicted to be a Janus kinase 1 (JAK1) inhibitor via molecular binding simulation. Mice with AD, established by repeated sensitization with 2,4-dinitrochlorobenzene (DNCB), were topically treated with PO hydrogel. Aurantiamide acetate was applied to HaCaT keratinocytes prior to inflammatory challenge in the presence or absence of JAK1-siRNA. Transcriptional and translational gene expressions associated with cutaneous inflammation or skin barriers were assessed by qPCR and Western blotting, respectively. Enzyme-linked immunosorbent assays were performed to detect immunoglobulin E and proinflammatory factors in skin tissue or serum. The phosphorylation of JAK1, signal transducer and activator of transcription (STAT)3, and STAT6 in keratinocytes and skin were analyzed by Western blotting.
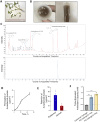
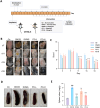
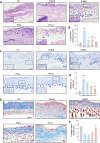
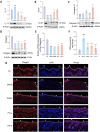
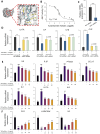
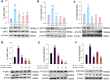
Results: In DNCB-sensitized mice, the PO hydrogel ameliorated skin lesions, lowered symptom scores, and reduced epidermal thickness by suppressing proinflammatory factor generation, oxidative stress, and the expression of CD4+. The PO hydrogel promoted the expression of caspase-14 and filaggrin, thereby helping restore skin barrier function in AD. The PO hydrogel and/or aurantiamide acetate inhibited the enzymatic activity of JAK1 and downstream (STAT)3/STAT6 signaling pathways in vitro and in vivo.
Conclusion: PO significantly ameliorated skin lesions and restored epidermal barrier function in AD mice. This was achieved by suppressing JAK1 enzymatic activity and JAK1-mediated STAT signaling pathways.
Keywords: JAK1 inhibition; aurantiamide acetate; cutaneous inflammation; epidermal barrier; filaggrin; purslane.
Copyright © 2025 Wei, Chen, Lai, Wang, Bian, Zhang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Taraxacum mongolicum Ameliorates DNCB-Induced Atopic Dermatitis-like Symptoms in Mice by Regulating Oxidative Stress, Inflammation, MAPK, and JAK/STAT/TSLP Signaling Pathways.Int J Mol Sci. 2025 Jul 9;26(14):6601. doi: 10.3390/ijms26146601. Int J Mol Sci. 2025. PMID: 40724851 Free PMC article.
-
Myricetin alleviates DNCB-induced atopic dermatitis by modulating macrophage M1/M2 polarization.Int Immunopharmacol. 2025 Jul 23;163:115212. doi: 10.1016/j.intimp.2025.115212. Online ahead of print. Int Immunopharmacol. 2025. PMID: 40706204
-
Hybrid Cannabis sativa L. inflorescences exert an anti-inflammatory effect through the modulation of MAPK/NF-κB/NLRP3 inflammasome and JAK1/STAT6 pathway in HaCaT cells.Front Pharmacol. 2025 Jul 24;16:1617180. doi: 10.3389/fphar.2025.1617180. eCollection 2025. Front Pharmacol. 2025. PMID: 40777996 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Interventions for preventing occupational irritant hand dermatitis.Cochrane Database Syst Rev. 2018 Apr 30;4(4):CD004414. doi: 10.1002/14651858.CD004414.pub3. Cochrane Database Syst Rev. 2018. PMID: 29708265 Free PMC article.
Cited by
-
Taraxacum mongolicum Ameliorates DNCB-Induced Atopic Dermatitis-like Symptoms in Mice by Regulating Oxidative Stress, Inflammation, MAPK, and JAK/STAT/TSLP Signaling Pathways.Int J Mol Sci. 2025 Jul 9;26(14):6601. doi: 10.3390/ijms26146601. Int J Mol Sci. 2025. PMID: 40724851 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

