Tissue plasminogen activator modified thromboelastography identifies fibrinolysis resistance in dogs with immune-mediated hemolytic anemia
- PMID: 40538729
- PMCID: PMC12177219
- DOI: 10.3389/fvets.2025.1571683
Tissue plasminogen activator modified thromboelastography identifies fibrinolysis resistance in dogs with immune-mediated hemolytic anemia
Abstract
Introduction: Immune-mediated hemolytic anemia (IMHA) is an important immunologic disorder in dogs that is associated with high mortality rates, frequently due to thromboembolism. Multiple factors contribute to the pathophysiology of thrombosis in IMHA including intravascular tissue factor expression, platelet activation, and neutrophil extracellular trap (NET) formation. It was hypothesized that dogs with IMHA have impaired fibrinolysis that can be detected using a modified viscoelastic assay and that biomarkers of NET formation are associated with this hypofibrinolysis.
Methods: Twenty dogs with non-associative IMHA were enrolled and paired thromboelastography (TEG) assays with and without additional tissue plasminogen activator (tPA) performed. A panel of hemostasis tests including measurement of plasma thrombin-activatable fibrinolysis inhibitor (TAFI) activity, active plasminogen activator inhibitor-1 (PAI-1), and concentrations of cell-free DNA (cfDNA) and nucleosomes were also performed.
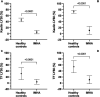
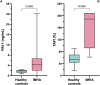
Results: Dogs with IMHA had hypercoagulable TEG tracings, increased TAFI activity and frequently displayed fibrinolysis resistance defined as minimal lysis in tPA augmented TEG assays. Increased concentrations of cfDNA, nucleosomes and active PAI-1 in dogs with IMHA compared to healthy controls were identified.
Discussion: These observations support the hypothesis that hypofibrinolysis is a common feature of IMHA in dogs. Increased plasma active PAI-1 concentrations and TAFI activities might contribute to the observed hypofibrinolysis. The combined hypercoagulability and hypofibrinolysis observed supports recent recommendations to provide thromboprophylaxis to all dogs with IMHA. These findings also suggest that NETosis might contribute to the common prothrombotic imbalance of IMHA in dogs.
Keywords: cell-free DNA; immunothrombosis; neutrophil extracellular traps; nucleosomes; plasminogen activator inhibitor-1; thrombin-activatable fibrinolysis inhibitor.
Copyright © 2025 Goggs, Davis and Brooks.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Thrombolysis for acute ischaemic stroke.Cochrane Database Syst Rev. 2003;(3):CD000213. doi: 10.1002/14651858.CD000213. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD000213. doi: 10.1002/14651858.CD000213.pub2. PMID: 12917889 Updated.
-
Immune-Mediated Hemolytic Anemia in Cats with Feline Infectious Peritonitis.Pathogens. 2025 Jul 4;14(7):660. doi: 10.3390/pathogens14070660. Pathogens. 2025. PMID: 40732707 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
References
-
- Weinkle TK, Center SA, Randolph JF, Warner KL, Barr SC, Erb HN. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemia in dogs: 151 cases (1993–2002). J Am Vet Med Assoc. (2005) 226:1869–80. doi: 10.2460/javma.2005.226.1869, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

