The mechanobiology of fibroblast activation in disease
- PMID: 40538750
- PMCID: PMC12178607
- DOI: 10.1063/5.0272393
The mechanobiology of fibroblast activation in disease
Abstract
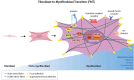
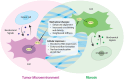
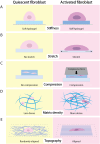
Fibroblasts play crucial roles in wound healing, cancer, and fibrosis. Many aspects of these roles are driven by the process known as fibroblast activation. The generally accepted definition of fibroblast activation is the transition from a quiescent state to a state in which fibroblasts participate in a number of active processes, including extracellular matrix (ECM) production and remodeling, elevated contractility, and enhanced migratory capacity, although there is no universal consensus on what exactly constitutes "activation." Interestingly, the time scale of activation is not consistent across tissues and disease states; some fibroblasts quickly return to quiescence after activation (e.g., in wound healing), others undergo apoptosis, while a subset become persistently activated. This activation, both acute and persistent, is inherently a mechanical process, given the increase in ECM production and remodeling and the enhanced traction force generation. Thus, there exists a dynamic reciprocity, or cell-ECM feedback, in which activated fibroblasts produce a mechanical microenvironment that in turn supports persistent activation. This has a wide variety of implications for disease, most notably fibrosis and cancer, as the fibroblasts that become persistently activated in connection with these conditions can contribute to disease state progression. Like other mechanosensitive processes, this mechanically induced persistent fibroblast activation is driven by a number of mechanotransduction signaling pathways. Thus, an opportunity exists in which the mechanosensitive underpinning of fibroblast activation can be leveraged to improve clinical outcomes. Here, we highlight these opportunities and make a call to the field to consider the mechanosensitive pathways governing fibroblast activation as an important frontier in mechanomedicine.
© 2025 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

Similar articles
-
Interventions targeted at women to encourage the uptake of cervical screening.Cochrane Database Syst Rev. 2021 Sep 6;9(9):CD002834. doi: 10.1002/14651858.CD002834.pub3. Cochrane Database Syst Rev. 2021. PMID: 34694000 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Psychological and/or educational interventions for the prevention of depression in children and adolescents.Cochrane Database Syst Rev. 2004;(1):CD003380. doi: 10.1002/14651858.CD003380.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Dec 07;(12):CD003380. doi: 10.1002/14651858.CD003380.pub3. PMID: 14974014 Updated.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

