Near-infrared light-induced photothermal and immunotherapy system for lung cancer bone metastasis treatment with simultaneous bone repair
- PMID: 40538765
- PMCID: PMC12178743
- DOI: 10.1016/j.bioactmat.2025.06.008
Near-infrared light-induced photothermal and immunotherapy system for lung cancer bone metastasis treatment with simultaneous bone repair
Abstract
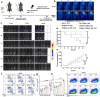
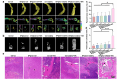
Approximately half of lung cancer patients experience bone metastasis, leading to bone loss, fracture, and other skeletal-related events. Although immunotherapies have significantly advanced the therapeutic landscape for lung cancer, bone metastases have an immunologically "cold" microenvironment, representing a challenging obstacle when treating lung cancer. The combination of immunotherapy and photothermal therapy (PTT) for treating tumor-induced bone defects holds promise for enhancing the efficacy of local tumor ablation and inhibiting tumor recurrence and metastasis through activating systemic immune responses. Herein, we developed an injectable hydrogel-based photothermal immunotherapy system (BP@Gel-CD[SA] hydrogel) incorporating STING agonists (SA) and black phosphorus nanosheets (BPNSs) for high-efficiency tumor elimination, immune activation, and bone regeneration. The photothermal and photodynamic activities of BPNSs induce hyperthermia and ROS-mediated apoptosis of tumor cells. Meanwhile, SA loaded into the nano-boxes in BP@Gel-CD[SA] hydrogel by host-guest interaction significantly activates the cGas-STING pathway. It stimulates immunogenic cell death (ICD), synergistically promoting immune cell infiltration. Single-cell RNA sequence analysis confirms the modulation of the tumor microenvironment (TME) through the PTT-mediated ICD effect and the transactivation of the cGAS-STING pathway in immune cells of the TME. More importantly, the system can significantly inhibit the growth of distant tumors via systemic immune activation and elicit long-term immune memory in addition to tumor eradication. In the long term, this hydrogel system can promote the formation of new bone at sites of tumor-induced bone destruction, improving bone strength in the affected area. Collectively, this strategy provides an effective and safe option for treating lung cancer bone metastases.
Keywords: Black phosphorus; Bone metastasis; Photothermal immunotherapy; STING activation.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










Similar articles
-
Enhanced antitumor immunity of VNP20009-CCL2-CXCL9 via the cGAS/STING axis in osteosarcoma lung metastasis.J Immunother Cancer. 2025 Jul 1;13(7):e012269. doi: 10.1136/jitc-2025-012269. J Immunother Cancer. 2025. PMID: 40592739 Free PMC article.
-
Lovastatin/SN38 co-loaded liposomes amplified ICB therapeutic effect via remodeling the immunologically-cold colon tumor and synergized stimulation of cGAS-STING pathway.Cancer Lett. 2024 Apr 28;588:216765. doi: 10.1016/j.canlet.2024.216765. Epub 2024 Feb 24. Cancer Lett. 2024. PMID: 38408604
-
Dual-modality immune nano-activator harnessing Mn2⁺ and quercetin to potentiate the cGAS-STING pathway for advanced cancer metalloimmunotherapy.J Nanobiotechnology. 2025 Mar 25;23(1):248. doi: 10.1186/s12951-025-03336-8. J Nanobiotechnology. 2025. PMID: 40128784 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Abbott A.G., Meyers D.E., Elmi-Assadzadeh G., Stukalin I., Marro A., Puloski S.K.T., Morris D.G., Cheung W.Y., Monument M.J. Effectiveness of immune checkpoint inhibitor therapy on bone metastases in non-small-cell lung cancer. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1379056. - DOI - PMC - PubMed
-
- Zhu Y.-J., Chang X.-S., Zhou R., Chen Y.-D., Ma H.-C., Xiao Z.-Z., Qu X., Liu Y.-H., Liu L.-R., Li Y., Yu Y.-Y., Zhang H.-B. Bone metastasis attenuates efficacy of immune checkpoint inhibitors and displays “cold” immune characteristics in Non-small cell lung cancer, Lung Cancer Amst. Neth. 2022;166:189–196. doi: 10.1016/j.lungcan.2022.03.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

