Dysbiosis of Gut Microbiota in Ankylosing Spondylitis Patients
- PMID: 40538777
- PMCID: PMC12178261
- DOI: 10.2147/JIR.S517979
Dysbiosis of Gut Microbiota in Ankylosing Spondylitis Patients
Abstract
Purpose: Ankylosing spondylitis (AS) is a chronic inflammatory disease associated with genetic, immune, and microbial factors. The role of gut microbiota in the pathogenesis of AS is increasingly recognized, with studies suggesting that intestinal dysfunction may trigger systemic inflammation. This study aimed to investigate the gut microbiota profiles of AS patients from Southern China and explore the relationship between gut microbiota and the occurrence and development of AS.
Patients and methods: We enrolled 30 AS patients and 25 healthy controls from the Fifth Affiliated Hospital of Sun Yat-sen University. Fecal samples were collected, and DNA was extracted for 16S rDNA sequencing to analyze the V3-V4 variable regions. Bioinformatic processing and statistical analysis were performed to assess the microbial community structure, diversity, and function.
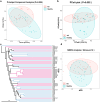
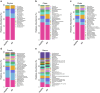
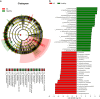
Results: The study revealed significant differences in gut microbiota composition between AS patients and healthy controls. AS patients exhibited a decrease in beneficial bacteria such as Firmicutes and Actinobacteria and an increase in harmful bacteria like Proteobacteria and Enterobacteriaceae. The functional prediction of gut microbiota indicated significant metabolic pathway alterations, particularly in energy metabolism, degradation metabolism, and nucleotide metabolism, which may be linked to the pathophysiology of AS.
Conclusion: This study demonstrates that the gut microbiota of Han Chinese AS patients in Guangdong Province is characterized by a decrease in beneficial bacterial communities and an increase in harmful ones, potentially contributing to AS progression through intestinal barrier disruption and intensified inflammatory responses. These findings provide a theoretical basis for developing new intervention strategies targeting the gut microbiota.
Keywords: 16S rDNA sequencing; abundance; inflammation; metabolic pathways.
© 2025 Yang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Integrating Gut Microbiome and Metabolomics with Magnetic Resonance Enterography to Advance Bowel Damage Prediction in Crohn's Disease.J Inflamm Res. 2025 Jun 11;18:7631-7649. doi: 10.2147/JIR.S524671. eCollection 2025. J Inflamm Res. 2025. PMID: 40535353 Free PMC article.
-
Radiation-induced injury and the gut microbiota: insights from a microbial perspective.Therap Adv Gastroenterol. 2025 Jun 16;18:17562848251347347. doi: 10.1177/17562848251347347. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40535532 Free PMC article. Review.
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
-
Characteristics of gut microbiota in patients with asthenozoospermia: a Chinese pilot study.BMC Microbiol. 2024 Jan 15;24(1):22. doi: 10.1186/s12866-023-03173-5. BMC Microbiol. 2024. PMID: 38225541 Free PMC article.
-
Exploring the potential mechanism of tofacitinib therapy for ankylosing spondylitis through gut microbiome and plasma metabolomics.Clin Rheumatol. 2025 Jul;44(7):2793-2808. doi: 10.1007/s10067-025-07467-z. Epub 2025 May 29. Clin Rheumatol. 2025. PMID: 40439987
References
LinkOut - more resources
Full Text Sources
Research Materials

