Endoplasmic reticulum-mitochondria crosstalk: new mechanisms in the development of atherosclerosis
- PMID: 40538812
- PMCID: PMC12176567
- DOI: 10.3389/fendo.2025.1573499
Endoplasmic reticulum-mitochondria crosstalk: new mechanisms in the development of atherosclerosis
Abstract
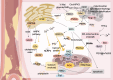
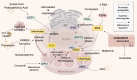
Atherosclerosis (AS) is a global public health concern and involves a complex pathogenesis characterized by lipid abnormalities, oxidative stress, and inflammatory responses at the cellular and molecular levels. The crosstalk between the endoplasmic reticulum (ER) and mitochondria, mediated by mitochondria-associated membranes (MAMs), plays a critical role in the pathogenesis of atherosclerosis. As two key cellular organelles, the ER and mitochondria interact physically and functionally through MAMs, which serve as bridges between their close contact and interdependence. MAMs maintain lipid homeostasis, promote calcium ion transport, the oxidative stress response, apoptosis, and autophagy. Recent studies have highlighted the significance of ER-mitochondria crosstalk in the progression of AS, as indicated by mitochondrial and ER structural and functional integrity, redox homeostasis, and calcium homeostasis. This review comprehensively explores the novel mechanisms of ER-mitochondria crosstalk in AS and emphasizes the potential of MAMs as therapeutic targets, aiming to provide new perspectives and strategies for the treatment of cardiovascular diseases.
Keywords: atherosclerosis; endoplasmic reticulum; endoplasmic reticulum contact complex; endoplasmic reticulum-mitochondrial crosstalk; mitochondria; mitochondria-associated membranes (MAMs).
Copyright © 2025 Li, Xiao, Dai, Chen, Pei and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The correlation between mitochondria-associated endoplasmic reticulum membranes (MAMs) and Ca2+ transport in the pathogenesis of diseases.Acta Pharmacol Sin. 2025 Feb;46(2):271-291. doi: 10.1038/s41401-024-01359-9. Epub 2024 Aug 8. Acta Pharmacol Sin. 2025. PMID: 39117969 Review.
-
Dendrobine attenuates lipopolysaccharide-induced acute lung injury by modulating FAM134B-mediated endoplasmic reticulum autophagy and mitochondrial function.Phytomedicine. 2025 Aug;144:156952. doi: 10.1016/j.phymed.2025.156952. Epub 2025 Jun 5. Phytomedicine. 2025. PMID: 40532488
-
Role of Mitochondria-Associated ER in Apoptosis.Cell Biochem Funct. 2025 Jul;43(7):e70105. doi: 10.1002/cbf.70105. Cell Biochem Funct. 2025. PMID: 40693348 Review.
-
The role of mitochondria-associated ER membranes in disease pathology: protein complex and therapeutic targets.Front Cell Dev Biol. 2025 Jun 30;13:1629568. doi: 10.3389/fcell.2025.1629568. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40661148 Free PMC article. Review.
-
Mitochondria-Associated Endoplasmic Reticulum Membranes in Human Health and Diseases.MedComm (2020). 2025 Jun 27;6(7):e70259. doi: 10.1002/mco2.70259. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40584408 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

