Imaging-based assessment of response to olaparib in platinum-sensitive relapsed ovarian cancer patients
- PMID: 40538854
- PMCID: PMC12176557
- DOI: 10.3389/fonc.2025.1546324
Imaging-based assessment of response to olaparib in platinum-sensitive relapsed ovarian cancer patients
Abstract
Background: High-grade serous carcinoma is a highly metastatic disease with a limited longterm disease control from systemic anti-cancer treatment, for which the radiological treatment response assessment metrics are imprecise. In this work, we developed noninvasive imagingbased measurements of spatial and longitudinal heterogeneity in a retrospective analysis of a phase 2 non-randomized study of germline BRCA1/BRCA2 mutated (gBRCAm) ovarian cancer patients treated with combination of PARP inhibitors (PARPi) and immune checkpoint inhibitors (ICIs).
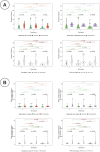
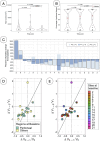
Methods: Lesions identified in CT images at baseline, week 4 (after PARPi only) and week 12 (after 8 weeks of PARPi + ICIs) were manually segmented. Anatomical networks of the metastatic sites were constructed to represent patterns of disease distribution. Volume and first-order radiomic features were computed and compared to different assessments of treatment response.
Results: The average number of edges per patient in the anatomical networks and total volumetric burden decreased with treatment were measured, differentiating between responders and nonresponders. Changes in volume at week 4 provided better indication of long-term response than the default RECIST assessment at the same time-point. Significant differences were also found between responders and non-responders in the first-order radiomic feature Energy.
Conclusions: In this feasibility study, we have demonstrated that noninvasive image-based analysis can identify quantitative imaging features associated with the response to the combination of PARPi and ICIs. These can be used to identify markers of response to ICIs from negative trials of a disease with limited response to ICIs.
Keywords: PARP inhibitors; computed tomography; immunotherapy; ovarian cancer; radiomics.
Copyright © 2025 Delgado-Ortet, Bura, Funingana, Hulse, Rundo, Brenton, Sala and Escudero Sanchez.
Conflict of interest statement
JB: Tailor Bio co-founder, stock options, AstraZeneca consulting and advisory roles, honoraria, Clovis Oncology consulting and advisory roles, GSK honoraria, holder of patents TAm-Seq v2 method for ctDNA estimation; enhanced detection of target DNA by fragment size analysis; methods for predicting treatment response in cancers. ES: Lucida Medical co-founder and shareholder, GE HealthCare research support, speakers’ bureau, Siemens, AWS and Philips Canon speakers’ bureau. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2022 Feb 16;2(2):CD007929. doi: 10.1002/14651858.CD007929.pub4. Cochrane Database Syst Rev. 2022. PMID: 35170751 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
References
-
- Cancer Research UK (CRUK) . Statistics by cancer type (ovarian-cancer). (2013) Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... (Accessed July 2023).
LinkOut - more resources
Full Text Sources
Miscellaneous

