Evaluation of a Digital Health Model of Care for the Management of Adults With Symptomatic Malignant Pleural Effusion
- PMID: 40538923
- PMCID: PMC12176497
- DOI: 10.1002/rcr2.70194
Evaluation of a Digital Health Model of Care for the Management of Adults With Symptomatic Malignant Pleural Effusion
Erratum in
-
Correction to "Evaluation of a Digital Health Model of Care for the Management of Adults With Symptomatic Malignant Pleural Effusion".Respirol Case Rep. 2025 Jul 18;13(7):e70273. doi: 10.1002/rcr2.70273. eCollection 2025 Jul. Respirol Case Rep. 2025. PMID: 40686527 Free PMC article.
Abstract
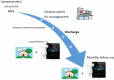
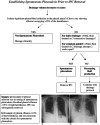
Background: The management of malignant pleural effusion (MPE) is inconsistent across health services. Many centres do not routinely offer all treatment options for MPE, with indwelling pleural catheter (IPC) being a primary example. This may be due to lack of specialist expertise or nursing capability to support the community-based treatment. New approaches are required to improve access to MPE treatments. This proof-of-concept study examines the feasibility of a virtual model of care for MPE, known as the specialist ambulatory pleural service (SAPS) model of care. This model will be compared with current approaches at other health services in the state, in terms of healthcare utilisation and costs. It will also assess health-related quality of life in individuals with MPE and report patient, carer and nurse experiences with the SAPS model of care.
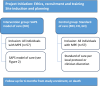
Methods: A prospective, multi-centre, mixed-methods study will be performed. Participants with symptomatic MPE requiring intervention will be consecutively enrolled. The primary outcome is pleural effusion-related hospitalisation from enrolment to death or end of study participation. Secondary outcomes include: Overall hospitalisation, unplanned pleural effusion-related outpatient and emergency department (ED) visits, pleural-related healthcare costs, adverse events, overall survival, percentage of screened patients recruited, percentage dropped out/lost to follow up, percentage of scheduled home visits carried out, percentage of teleultrasound assessments completed, technical issues, percentage of symptom logbooks completed, quality of life, longitudinal symptom monitoring, participant and nursing attitudes to the SAPS model of care, patient activation measure and a stakeholder interview of the SAPS model of care implementation.
Discussion: Digital health may improve access to MPE treatments by reducing barriers to specialist care and facilitating training and support for community staff. This trial assesses the SAPS model of care, providing data on barriers and facilitators to its implementation, its efficacy, costs and qualitative outcomes. Trial Registration: Australia New Zealand Clinical Trial Registry: ACTRN12623000063617; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=384448&isReview=true.
Keywords: digital health; malignant pleural effusion; nursing care; patient‐reported outcomes; teleultrasound.
© 2025 The Author(s). Respirology Case Reports published by John Wiley & Sons Australia, Ltd on behalf of The Asian Pacific Society of Respirology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
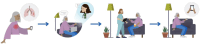
Comment in
-
Correction to "Evaluation of a Digital Health Model of Care for the Management of Adults With Symptomatic Malignant Pleural Effusion".Respirol Case Rep. 2025 Jul 18;13(7):e70273. doi: 10.1002/rcr2.70273. eCollection 2025 Jul. Respirol Case Rep. 2025. PMID: 40686527 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

