Remediation of acetochlor-contaminated maize field soil using Serratia odorifera AC-1 fertilizer: effects on soil microbial communities
- PMID: 40539105
- PMCID: PMC12177894
- DOI: 10.3389/fmicb.2025.1510157
Remediation of acetochlor-contaminated maize field soil using Serratia odorifera AC-1 fertilizer: effects on soil microbial communities
Abstract
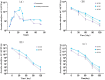
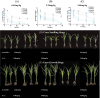
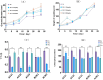
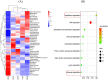
Acetochlor is a chloroacetamide herbicide that is widely applied in corn fields. Nevertheless, the long-term usage of acetochlor in the soil leads to residues, which severely affect the germination of corn seeds and the growth of seedlings, and even exert an influence on the soil microbial community. Microbial degradation of acetochlor is the principal approach for restoring the soil microbial ecology. In this study, the Serratia odorifera AC-1 strain was isolated and identified from the soil for the degradation of residual acetochlor in the soil. To enhance the degradation efficiency, a solid microbial agent was prepared by using activated carbon as a carrier and the AC-1 strain at a 1:1 ratio and applied to the soil for degradation and remediation experiments. The content of the microbial cells in the solid microbial agent was 1.49 × 106 CFU/g after 120 days of preparation. The application of the AC-1 solid microbial agent significantly influenced the relative abundance of soil microbial communities (Actinobacteria, Firmicutes, and Proteobacteria), increasing the diversity of bacterial populations in the soil. The experimental results indicated that after the application of the AC-1 solid microbial agent, the plant height, stem diameter, and photosynthetic efficiency of corn seedlings under acetochlor stress were significantly elevated. When the application rate of the AC-1 solid microbial agent was 5.00 mg/kg, the stem diameter of corn increased by 56.4% compared with the control group. When the acetochlor concentration in the soil was 6.65 mg/kg, the DT50 value of the AC-1 solid microbial agent was 2.28 days. This study clarified the degradation mechanism and remediation capacity of the Serratia odorifera AC-1 strain in acetochlor-contaminated soil and proposed a new strategy to improve the stability and degradation efficiency of the microbial strain by optimizing the immobilization technology of the strain on activated carbon. This research provides a scientific basis and technical guidance for the future application of bioremediation technology in the field environment to remove pesticide residues, restore soil health, and enhance crop productivity.
Keywords: Serratia odorifera; acetochlor; bioremediation; seed germination; soil microbial communities.
Copyright © 2025 Zhang, Shi, Zheng and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
[Serratia marcescens TF-1 for biodegradation of chlorobenzene contaminants in soil and its application in in-situ remediation of chemical industrial sites].Sheng Wu Gong Cheng Xue Bao. 2025 Jun 25;41(6):2483-2497. doi: 10.13345/j.cjb.241011. Sheng Wu Gong Cheng Xue Bao. 2025. PMID: 40550684 Chinese.
-
Possible Use in Soil Bioremediation of the Bacterial Strain Bacillus Sphaericus NM-1 Capable of Simultaneously Degrading Promethrin and Acetochlor.Microorganisms. 2025 Jul 19;13(7):1698. doi: 10.3390/microorganisms13071698. Microorganisms. 2025. PMID: 40732207 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.Health Technol Assess. 2008 Jun;12(28):iii-iv, ix-95. doi: 10.3310/hta12280. Health Technol Assess. 2008. PMID: 18547499
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
References
-
- Desoky E. S. M., Saad A. M., el-Saadony M. T., Merwad A. R. M., Rady M. M. (2020). Plant growth-promoting rhizobacteria improve antioxidant defense system and suppress oxidative stress in wheat under salinity. Biocatal. Agric. Biotechnol. 30:101878. doi: 10.1016/j.bcab.2020.101878 - DOI
-
- Dong M., Xiao Y., Yang B., Wang S., Sun L., Han Z., et al. (2024). Serratia marcescens AB1: a rhizosphere bacterium mitigating the acetochlor stress on the soil environment. Rhizosphere 30:100898. doi: 10.1016/j.rhisph.2024.100898 - DOI
-
- El-Esawi M. A., Alaraidh I. A., Alsahli A. A., Alzahrani S. M., Ali H. M., Alayafi A. A., et al. (2018). Serratia liquefaciens KM4 improves salt stress tolerance in maize by regulating redox potential and stress-related gene expression. Int. J. Mol. Sci. 19:3310. doi: 10.3390/ijms19113310, PMID: - DOI - PMC - PubMed
-
- Gu X., Zheng L., Zhai Q., Sun J., He H., Tang H., et al. (2022). Optimization of fermentation medium for biocontrol strain Pantoea jilinensis D25 and preparation of its microcapsules. Process Biochem. 121, 216–227. doi: 10.1016/j.procbio.2022.07.006 - DOI
LinkOut - more resources
Full Text Sources

