Assessment of Anticancer Effects of Aloe vera on 3D Liver Tumor Spheroids in a Microfluidic Platform
- PMID: 40539369
- PMCID: PMC12322640
- DOI: 10.1002/bit.29033
Assessment of Anticancer Effects of Aloe vera on 3D Liver Tumor Spheroids in a Microfluidic Platform
Abstract
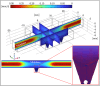
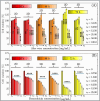
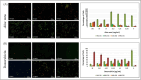
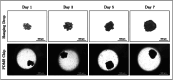
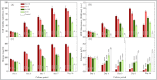
The search for effective anticancer therapies has increasingly focused on natural compounds like Aloe vera, renowned for its therapeutic properties. This study investigates the anticancer properties of Aloe vera on 3D liver tumor spheroids via a PDMS-based microfluidic device, providing a more physiologically realistic model compared to traditional 2D cultures. HepG2 cells were cultivated to generate 3D spheroids on-chip, thereafter subjected to different concentrations of Aloe vera and the chemotherapeutic drug Doxorubicin to evaluate cytotoxic effects. The microfluidic system, validated by COMSOL simulations, facilitated continuous perfusion and real-time assessment of cell viability over a duration of 10 days. The results indicated that Aloe vera markedly diminished cell viability by triggering apoptosis at concentrations over 12.5 mg/mL. IC50 values were determined at 72 h: 25 ± 0.10 mg/mL for Aloe vera and 5.47 ± 0.03 µg/mL for Doxorubicin in 2D cultures, but in 3D cultures, the IC50 values were 31.25 ± 0.14 mg/mL for Aloe vera and 8.33 ± 0.05 µg/mL for Doxorubicin. This study underscores the promise of Aloe vera as a natural anticancer agent and illustrates the efficacy of microfluidic platforms for enhanced drug screening and customized medicine applications.
Keywords: 3D cell culture; Aloe vera; anticancer agent; microfluidic.
© 2025 The Author(s). Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Algarni, A. A. 2021. “Cytotoxic Activity of Ethanolic Extract of Aloe Saudiarabica and Aloe Shadensis Against Three Human Cancer Cell Line.” Journal of Pharmaceutical Research International 1: 138–146.
-
- Ashutosh Kumar Yadav, Y. , Abhishek Yadav Y., Manish Yadav Y., Md Akhlak A., Shweta Mishra M., and Jitendra Kumar Rai R.. 2023. “A Review on Drug Stability.” International Journal of Science and Research Archive 9: 474–485.
-
- Bagherian, T. , Tackallou S. H., and Mohammadgholi A.. 2021. “Quantitative Measurement of Bax and Bcl2 Genes and Protein Expression in MCF7 Cell‐Line When Treated by Aloe vera Extract.” Gene Reports 23: 101123. https://www.sciencedirect.com/science/article/pii/S2452014421001084.
-
- Bakshi, H. A. , Mkhael M., Faruck H. L., et al. 2024. “Cellular Signaling In the Hypoxic Cancer Microenvironment: Implications for Drug Resistance and Therapeutic Targeting.” Cellular Signalling 113: 110911. https://www.sciencedirect.com/science/article/pii/S0898656823003261. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

