Hydrophobic and Polarized Aromatic Residues Promote Internalization of Arg-Rich Cell-Penetrating Peptides through Ionpair-π Interactions
- PMID: 40539411
- PMCID: PMC12284625
- DOI: 10.1002/chem.202501138
Hydrophobic and Polarized Aromatic Residues Promote Internalization of Arg-Rich Cell-Penetrating Peptides through Ionpair-π Interactions
Abstract
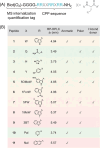
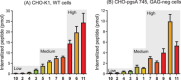
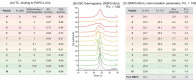
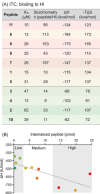
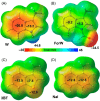
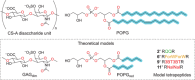
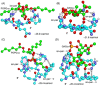
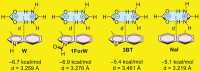
Cell penetrating peptides (CPPs) are small sequences that can cross cell membranes. Arg and Trp are highly prevalent amino acids in natural and synthetic efficient CPP sequences. In particular, Trp is essential and cannot be substituted by other hydrophobic or aromatic amino acids. The aim of the present study is to decipher the role of Trp in synthetic Arg/Trp CPP sequences. To do so, a small peptide library in which this residue was substituted by other natural or nonnatural amino acids was designed. Internalization of these peptides in cells was evaluated, and it appeared that combining aromaticity and hydrophobicity in the presence of Arg residues leads to enhanced internalization. The study of the interaction of these peptides with model lipid membranes revealed that the modulation of hydrophobicity promoted insertion in bilayers but had little impact on the binding affinity. On the other hand, more hydrophobic substitutes of Trp led to more favorable binding enthalpies to heparin. With density functional theory (DFT) analysis, we suggest that ion-pair···π interactions between the aromatic ring and the ion pair formed by the positively charged Arg and the negatively charged cell surface groups can be established and could be at the origin of the unique internalization properties of Trp-containing Arg-rich CPPs.
Keywords: DFT; cell‐penetrating peptides; glycosaminoglycans; tryptophan.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ionpair-π interactions favor cell penetration of arginine/tryptophan-rich cell-penetrating peptides.Biochim Biophys Acta Biomembr. 2020 Feb 1;1862(2):183098. doi: 10.1016/j.bbamem.2019.183098. Epub 2019 Oct 30. Biochim Biophys Acta Biomembr. 2020. PMID: 31676372
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Adapting Safety Plans for Autistic Adults with Involvement from the Autism Community.Autism Adulthood. 2025 May 28;7(3):293-302. doi: 10.1089/aut.2023.0124. eCollection 2025 Jun. Autism Adulthood. 2025. PMID: 40539213
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Derossi D., Joliot A. H., Chassaing G., Prochiantz A., J. Biol. Chem. 1994, 269, 10444. - PubMed
-
- Vivès E., Brodin P., Lebleu B., J. Biol. Chem. 1997, 272, 16010. - PubMed
-
- Mitchell D. J., Steinman L., Kim D. T., Fathman C. G., Rothbard J. B., J. Pept. Res. 2000, 56, 318. - PubMed
-
- Futaki S., Suzuki T., Ohashi W., Yagami T., Tanaka S., Ueda K., Sugiura Y., J. Biol. Chem. 2001, 276, 5836. - PubMed
-
- Gehring W. J., Affolter M., Bürglin T., Annu. Rev. Biochem. 1994, 63, 487. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

