Recombinant chromosome 6 open reading frame 120 protein promotes angiogenesis and endothelial‑to‑mesenchymal transition in human umbilical vein endothelial cells via the PI3K/Akt signaling pathway
- PMID: 40539464
- PMCID: PMC12203039
- DOI: 10.3892/mmr.2025.13596
Recombinant chromosome 6 open reading frame 120 protein promotes angiogenesis and endothelial‑to‑mesenchymal transition in human umbilical vein endothelial cells via the PI3K/Akt signaling pathway
Abstract
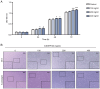
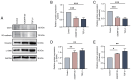
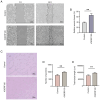
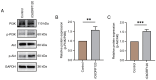
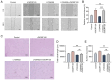
The vascular endothelium plays a pivotal role in modulating various physiological processes and its dysfunction is fundamental to the development of numerous vascular and non‑vascular diseases. Chromosome 6 open reading frame 120 (C6ORF120) has been implicated in cellular processes such as apoptosis, inflammation, immunomodulation and fibrosis. However, the specific effects of C6ORF120 on endothelial cell function remain unclear. The present study aimed to explore the potential role of C6ORF120 in endothelial dysfunction and its underlying molecular mechanisms. It synthesized recombinant C6ORF120 protein (rC6ORF120) and assessed its effects on human umbilical vein endothelial cells (HUVECs) through various functional assays, including the CCK‑8 assay for proliferation, scratch assay for migration and tube formation assay for angiogenesis. Additionally, immunofluorescence (IF) and western blotting (WB) were employed to evaluate endothelial‑mesenchymal transition (EndMT). The present study also quantified the expression of key proteins within the PI3K/Akt signaling pathway to elucidate its role in mediating the effects of rC6ORF120 on HUVECs. Treatment with rC6ORF120 significantly enhanced HUVEC proliferation (200 ng/ml vs. control at 72 h, 1.14±0.01 vs. 1.05±0.02; t=8.15; P<0.001) and induced phenotypic changes. In migration and angiogenesis assays, rC6ORF120‑treated HUVECs exhibited increased wound closure (37.69±2.74% vs. 66.16±6.13%; t=7.35; P=0.002) and angiogenesis assays showed significant improvements in tube formation parameters such as total tubule length (77,199.67±4,684.88 µm vs. 96,203.00±3,354.89 µm; t=5.71; P=0.002). WB and IF analyses both indicated that rC6ORF120 promotes EndMT in HUVECs. Furthermore, rC6ORF120 treatment increased PI3K/Akt phosphorylation significantly compared with controls (p‑PI3K; 1.57±0.18 vs. 1.00±0.00; t=5.64; P=0.005). LY294002 significantly reversed these effects on EndMT and angiogenesis (P<0.05), while the effect on cell migration was less pronounced (P=0.565). Our study highlights the critical role of C6ORF120 in HUVECs, promoting proliferation, migration, angiogenesis and EndMT, which are mediated, at least in part, by the PI3K/Akt pathway.
Keywords: PI3K/Akt; chromosome 6 open reading frame 120; endothelial dysfunction; endothelial‑mesenchymal transition; human umbilical vein endothelial cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Adipose-derived stem cells extracellular vesicles enhance diabetic wound healing via CCN2/PI3K/AKT pathway: therapeutic potential and mechanistic insights.Stem Cell Res Ther. 2025 Jun 15;16(1):304. doi: 10.1186/s13287-025-04354-x. Stem Cell Res Ther. 2025. PMID: 40518546 Free PMC article.
-
Quinolinone Derivatives Suppress Angiogenesis in Human Umbilical Vein Endothelial Cells by Blocking the VEGF-Induced VEGFR2 Signaling Pathway.Arch Pharm (Weinheim). 2025 Jul;358(7):e70040. doi: 10.1002/ardp.70040. Arch Pharm (Weinheim). 2025. PMID: 40590182
-
Estradiol Downregulates MicroRNA-193a to Mediate Its Angiogenic Actions.Cells. 2025 Jul 23;14(15):1134. doi: 10.3390/cells14151134. Cells. 2025. PMID: 40801567 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

