Real-World Improvements of Lung Clearance Index and Ventilation Distribution Efficiency in Children With Cystic Fibrosis After Elexacaftor/Tezacaftor/Ivacaftor Initiation
- PMID: 40539675
- PMCID: PMC12180290
- DOI: 10.1002/ppul.71166
Real-World Improvements of Lung Clearance Index and Ventilation Distribution Efficiency in Children With Cystic Fibrosis After Elexacaftor/Tezacaftor/Ivacaftor Initiation
Abstract
Introduction: Elexacaftor/tezacaftor/ivacaftor (ETI) is a breakthrough therapy for cystic fibrosis (CF). We aimed to assess ETI's real-world impact on peripheral airway disease assessed as ventilation distribution inhomogeneity using nitrogen multiple breath washout (N2MBW) in children aged 6-17 years. Additionally, we compared the two outcomes, lung clearance index (LCI), and ventilation distribution efficiency (VDE), as VDE is considered to adjust for a theoretical overestimation of lung disease when using LCI.
Methods: This nationwide study included data from N2MBW performed during routine clinical care. Linear mixed effect regression was used to assess changes in LCI and VDE after 12 months of ETI treatment. Subgroup analyses included baseline age (6-11 vs. 12-1 years) and disease severity (normal-moderate vs. severe-very severe).
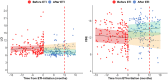
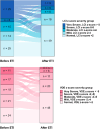
Results: We included 131 children (78% homozygous for F508del mutation, mean [SD] age 11.5 [3.4]), and 339 N2MBW tests. The median (range) number of tests per child was 3 (1-10). The estimated mean (95% CI) 12-months post-ETI improvement in LCI and VDE were 1.7 units (-2.1; -1.2, p < 0.001) and 2.1%-point (1.6; 2.6, p < 0.001), respectively. Similar LCI and VDE improvements were observed across age groups. Using VDE, fewer children were categorized with very severe lung disease, and the ETI-effect did not differ between the severity groups, unlike LCI.
Conclusion: Our research demonstrates that ETI treatment significantly improves lung function, as measured by N2MBW, in Danish children and adolescents with CF. VDE improvements were consistent across age and disease severity groups. In contrast, LCI revealed larger effect estimates for those with severe to very-severe lung impairment.
Keywords: LCI; VDE; cystic fibrosis; elexacaftor/tezacaftor/ivacaftor; real world study.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
K.G. Nielsen reports advisory board membership for Boehringer Ingelheim and Recode Therapeutics and is current member of the ATS Inert Gas Washout Technical Standard Task Force.
Figures


References
-
- Shteinberg M., Haq I. J., Polineni D., and Davies J. C., “Cystic Fibrosis,” Lancet 397, no. 10290 (2021): 2195–2211. - PubMed
-
- Elborn J. S., “Cystic Fibrosis,” Lancet 388, no. 10059 (2016): 2519–2531. - PubMed
-
- Qvist T., Nielsen B. U., Olesen H. V., et al., “Close Monitoring and Early Intervention: Management Principles for Cystic Fibrosis in Denmark,” APMIS 132 (January 2024): apm.13375. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

