Discovery and Validation of a Novel Class of Necroptosis Inhibitors Targeting RIPK1
- PMID: 40539767
- PMCID: PMC12281487
- DOI: 10.1021/acschembio.5c00112
Discovery and Validation of a Novel Class of Necroptosis Inhibitors Targeting RIPK1
Abstract
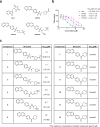
Necroptosis is a form of programmed cell death that, when dysregulated, is associated with cancer and inflammatory and neurodegenerative diseases. Here, starting from hits identified from a phenotypic high-throughput screen for inhibitors of necroptosis, we synthesized a library of compounds containing a 7-phenylquinoline motif and validated their anti-necroptotic activity in a novel live-cell assay. Based on these data, we designed an optimized photoaffinity probe for target engagement studies and through biochemical and cell-based assays established receptor-interacting kinase 1 (RIPK1) as the cellular target, with inhibition of necroptosis arising from the prevention of RIPK1 autophosphorylation and activation. X-ray crystallography and mass spectrometry revealed that these compounds bind at the hinge region of the active conformation of RIPK1, establishing them as type I kinase inhibitors. In addition, we demonstrated in vitro synergy with type III kinase inhibitors, such as necrostatin-1 and found that lead compounds protected mice against acute inflammation in necroptosis models in vivo. Overall, we present a novel pharmacophore for inhibition of human RIPK1, a key protein involved in necroptosis, and provide a photoaffinity probe to explore RIPK1 target engagement in cells.
Figures


References
-
- Fritsch M., Günther S. D., Schwarzer R., Albert M. C., Schorn F., Werthenbach J. P., Schiffmann L. M., Stair N., Stocks H., Seeger J. M., Lamkanfi M., Krönke M., Pasparakis M., Kashkar H.. Caspase-8 Is the Molecular Switch for Apoptosis, Necroptosis and Pyroptosis. Nature. 2019;575(7784):683–687. doi: 10.1038/s41586-019-1770-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

