Efficacy of oncolytic virus in the treatment of intermediate-to-advanced solid tumors: a systematic review and meta-analysis
- PMID: 40539780
- PMCID: PMC12282134
- DOI: 10.1128/jvi.00640-25
Efficacy of oncolytic virus in the treatment of intermediate-to-advanced solid tumors: a systematic review and meta-analysis
Abstract
This meta-analysis assessed oncolytic virus therapy's efficacy for intermediate-to-advanced solid tumors. We systematically searched PubMed, Cochrane Library, and Embase databases until March 6, 2025. Our review included 87 studies, involving 5,385 individuals, encompassing 75 clinical trials and 12 retrospective studies. Patient response rates were as follows: complete response (CR) 11%, partial response (PR) 18%, stable disease (SD) 32%, and progressive disease (PD) 32%. The overall response rate (ORR) was 29%, and the durable response rate (DRR) was 39%. Comparing oncolytic virus therapy to alternatives, the odds ratio (OR) for overall response was 1.62, and the hazard ratio (HR) for overall survival (OS) was 0.86. This analysis emphasizes the effectiveness of oncolytic virus therapy for intermediate-to-advanced solid tumors, highlighting its promise as a treatment option. These findings offer vital insights into cancer therapy researchers and practitioners.IMPORTANCEAlthough previous meta-analyses on this topic have been published, our research addresses the limitations of previous studies by including missed and newly published studies, updating the results, and conducting extensive subgroup analyses. Our study fills a gap in the literature by providing a comprehensive evaluation of the therapeutic potential of oncolytic viruses for solid tumors.
Keywords: intermediate-to-advanced solid tumor; meta-analysis; oncolytic virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
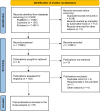


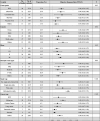


Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Next-generation sequencing for guiding matched targeted therapies in people with relapsed or metastatic cancer.Cochrane Database Syst Rev. 2025 Mar 24;3(3):CD014872. doi: 10.1002/14651858.CD014872.pub2. Cochrane Database Syst Rev. 2025. PMID: 40122129
-
Targeted therapy for advanced anaplastic lymphoma kinase (<I>ALK</I>)-rearranged non-small cell lung cancer.Cochrane Database Syst Rev. 2022 Jan 7;1(1):CD013453. doi: 10.1002/14651858.CD013453.pub2. Cochrane Database Syst Rev. 2022. PMID: 34994987 Free PMC article.
References
-
- Mozaffari Nejad AS, Fotouhi F, Mehrbod P, Keshavarz M, Alikhani MY, Ghaemi A. 2020. Oncolytic effects of Hitchner B1 strain of newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways. Microb Pathog 147:104438. doi: 10.1016/j.micpath.2020.104438 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

