Legionella pneumophila type II secretome reveals a polysaccharide deacetylase that impacts intracellular infection, biofilm formation, and resistance to polymyxin- and serum-mediated killing
- PMID: 40539790
- PMCID: PMC12239567
- DOI: 10.1128/mbio.01393-25
Legionella pneumophila type II secretome reveals a polysaccharide deacetylase that impacts intracellular infection, biofilm formation, and resistance to polymyxin- and serum-mediated killing
Erratum in
-
Erratum for Adams et al., "Legionella pneumophila type II secretome reveals a polysaccharide deacetylase that impacts intracellular infection, biofilm formation, and resistance to polymyxin- and serum-mediated killing".mBio. 2025 Aug 13;16(8):e0203425. doi: 10.1128/mbio.02034-25. Epub 2025 Jul 23. mBio. 2025. PMID: 40698934 Free PMC article. No abstract available.
Abstract
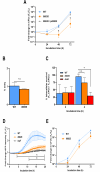
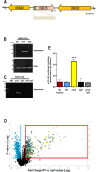
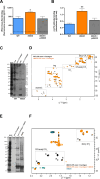
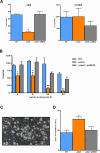
Prior analyses suggested that the type II secretion system (T2SS) of Legionella pneumophila secretes ≥47 proteins beyond its 26 known substrates. Upon examination of mutants of wild-type strain 130b that lack those exoproteins most conserved across the Legionella genus, we discovered that protein "06635" majorly promotes L. pneumophila replication within amoebae. Immunoblotting, proteomics, and whole-cell enzyme-linked immunosorbent assay (ELISA) confirmed that 06635 exists in culture supernatants and on the bacterial outer surface and does so in a T2SS-dependent manner. Bioinformatic analyses identified 06635 as a novel member of the carbohydrate esterase family 4, whose members deacetylate bacterial surface polysaccharides, peptidoglycan, chitins, and/or xylans. Given 06635's T2SS-dependent secretion, low-level amino acid similarity to known peptidoglycan deacetylases, and the unaltered lysozyme resistance of a 06635 mutant, we pursued the hypothesis that 06635 deacetylates a polysaccharide on L. pneumophila's surface. Supporting this, the 06635 mutant exhibited increased binding to both wheat germ agglutinin (i.e., more surface N-acetylglucosamine) and antibodies that recognize acetylated lipopolysaccharide (LPS). Nuclear magnetic resonance (NMR) analysis of isolated mutant vs wild-type LPS confirmed that 06635 promotes LPS deacetylation. Thus, we designated 06635 as PdaA, for
Keywords: L. pneumophila; PdaA; T2SS; autoaggregation; biofilm formation; intracellular infection; lipopolysaccharide; polysaccharide deacetylase; resistance to polymyxin B; serum-resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Versatile regulation of effectors by novel orthologous regulators in the Legionella genus.mBio. 2025 Jul 9;16(7):e0126825. doi: 10.1128/mbio.01268-25. Epub 2025 May 30. mBio. 2025. PMID: 40444465 Free PMC article.
-
The intracellular growth of the vacuolar pathogen Legionella pneumophila is dependent on the acyl chain composition of host membranes.bioRxiv [Preprint]. 2023 Nov 20:2023.11.19.567753. doi: 10.1101/2023.11.19.567753. bioRxiv. 2023. PMID: 38045297 Free PMC article. Preprint.
-
Optimized Legionella expression strain for affinity purification of His-tagged membrane proteins eliminates major multimeric contaminant.Microbiol Spectr. 2025 Jul;13(7):e0322224. doi: 10.1128/spectrum.03222-24. Epub 2025 May 19. Microbiol Spectr. 2025. PMID: 40387337 Free PMC article.
-
Legionella pneumophila, a Rosetta stone to understanding bacterial pathogenesis.J Bacteriol. 2024 Dec 19;206(12):e0032424. doi: 10.1128/jb.00324-24. Epub 2024 Dec 5. J Bacteriol. 2024. PMID: 39636264 Free PMC article. Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
-
- Hochstrasser R, Michaelis S, Brülisauer S, Sura T, Fan M, Maaß S, Becher D, Hilbi H. 2022. Migration of Acanthamoeba through Legionella biofilms is regulated by the bacterial Lqs-LvbR network, effector proteins and the flagellum. Environ Microbiol 24:3672–3692. doi: 10.1111/1462-2920.16008 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

