VLX600, an anticancer iron chelator, exerts antimicrobial effects on Mycobacterium abscessus infections
- PMID: 40539807
- PMCID: PMC12323642
- DOI: 10.1128/spectrum.00719-25
VLX600, an anticancer iron chelator, exerts antimicrobial effects on Mycobacterium abscessus infections
Abstract
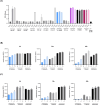
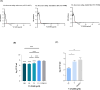
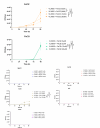
Mycobacterium abscessus presents significant clinical challenges due to its intrinsic and acquired resistance to antibiotics, resulting in prolonged treatments and poor patient outcomes. Addressing the urgent need for novel therapeutics, this study explores the antimicrobial potential of VLX600, originally developed as an anticancer agent, against M. abscessus. Screening a library of 3,200 clinically evaluated compounds identified VLX600 as a potent antimicrobial with minimal cytotoxicity. VLX600 demonstrated inhibitory effects against various strains of M. abscessus with minimum inhibitory concentrations of 4 µg/mL-16 µg/mL. It also remained effective in intracellular M. abscessus in host cells and exhibited broad-spectrum activity against other bacterial species, including Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The antimicrobial activity of VLX600 was abrogated by supplemental iron, indicating a mechanism dependent on iron chelation. VLX600 significantly reduced bacterial burdens and inflammation in a murine model of pulmonary M. abscessus infection. Additionally, synergistic effects were observed when VLX600 was combined with conventional antibiotics such as amikacin and clarithromycin in vitro. These findings highlight VLX600 as a promising candidate for repurposing as an antimicrobial agent against M. abscessus, warranting further clinical investigations.IMPORTANCEMycobacterium abscessus is an opportunistic pathogen that commonly causes pulmonary infections in cystic fibrosis patients. These infections are notoriously difficult to treat due to high levels of antibiotic resistance of M. abscessus, resulting in low cure rates. In this study, we identified a novel antibiotic candidate, VLX600, through high-throughput screening of 3,200 clinical compounds and demonstrated that VLX600 inhibits the growth of M. abscessus by depriving it of ferric and ferrous ions. This study highlights the potential of iron chelators as antimicrobial agents against M. abscessus infections. Since iron is an essential nutrient for the growth of many bacteria, the use of iron chelators could be extended to other infectious diseases. We hope this research will inspire further studies aimed at developing iron chelators as a novel class of antimicrobial agents.
Keywords: Mycobacterium abscessus; VLX600; antibiotics; drug repositioning; iron chelator.
Conflict of interest statement
J.-J. Yim has served as the overall or institutional principal investigator for clinical trials related to non-tuberculous mycobacterial pulmonary disease sponsored by LigaChem Biosciences, Insmed, and AN2 Therapeutics. Additionally, he has received several drugs free of charge as a principal investigator for previous trials related to tuberculosis from Pfizer, Otsuka, and Yuhan.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

