Photoaffinity Ligand of Cystic Fibrosis Corrector VX-445 Identifies SCCPDH as an Off-Target
- PMID: 40539870
- PMCID: PMC12281486
- DOI: 10.1021/acschembio.5c00157
Photoaffinity Ligand of Cystic Fibrosis Corrector VX-445 Identifies SCCPDH as an Off-Target
Abstract
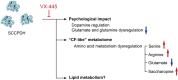
Cystic fibrosis (CF) pharmacological correctors improve the cystic fibrosis transmembrane conductance regulator (CFTR) protein trafficking and function. Several side effects of these correctors and adverse drug interactions have been reported, emphasizing the need to understand off-targets. We synthesized VU439, a functionalized photoaffinity ligand (PAL) of VX-445. Chemoproteomics analysis by mass spectrometry (MS) was used to identify cross-linked proteins in CF bronchial epithelial cells expressing F508del CFTR. We identified saccharopine dehydrogenase-like oxidoreductase (SCCPDH), an uncharacterized putative oxidoreductase, as a VX-445-specific off-target. We also characterized changes in the metabolomic profiles of cells overexpressing SCCPDH to determine the consequence of binding of VX-445 to SCCPDH. These data show dysregulation of amino acid metabolism and a potential inhibitory activity of VX-445 on SCCPDH. The identified off-target may explain the exacerbation of psychological symptoms observed in the clinic, thus emphasizing the need for further optimization of correctors.
Figures





Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2023 Nov 20;11(11):CD010966. doi: 10.1002/14651858.CD010966.pub4. Cochrane Database Syst Rev. 2023. PMID: 37983082 Free PMC article.
-
Structure-guided combination of novel CFTR correctors to improve the function of F508del-CFTR in airway epithelial cells.Biochem Pharmacol. 2025 Oct;240:117127. doi: 10.1016/j.bcp.2025.117127. Epub 2025 Jul 9. Biochem Pharmacol. 2025. PMID: 40645602
-
Ataluren and similar compounds (specific therapies for premature termination codon class I mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2023 Mar 3;3(3):CD012040. doi: 10.1002/14651858.CD012040.pub3. Cochrane Database Syst Rev. 2023. PMID: 36866921 Free PMC article.
-
Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease.Cochrane Database Syst Rev. 2016 Jun 17;2016(6):CD005599. doi: 10.1002/14651858.CD005599.pub5. Cochrane Database Syst Rev. 2016. PMID: 27314455 Free PMC article.
-
Antibiotic treatment for non-tuberculous mycobacteria lung infection in people with cystic fibrosis.Cochrane Database Syst Rev. 2025 Mar 27;3(3):CD016039. doi: 10.1002/14651858.CD016039. Cochrane Database Syst Rev. 2025. PMID: 40145528
References
-
- Rommens J. M., Kerem B., Alon N., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J., Drumm M. L., Iannuzzi M. C.. et al. Identification of the Cystic Fibrosis Gene : Cloning and Characterization of Complementary DNA. Science. 1989;245:1066–1073. doi: 10.1109/CMSBSE.2013.6605713. - DOI - PubMed
-
- Wainwright C. E., Elborn J. S., Ramsey B. W., Marigowda G., Huang X., Cipolli M., Colombo C., Davies J. C., De Boeck K., Flume P. A.. et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2015;373:220. doi: 10.1056/NEJMoa1409547. - DOI - PMC - PubMed
-
- Keating D., Marigowda G., Burr L., Daines C., Mall M. A., McKone E. F., Ramsey B. W., Rowe S. M., Sass L. A., Tullis E.. et al. VX-445–Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018;379:1612–1620. doi: 10.1056/NEJMoa1807120. - DOI - PMC - PubMed
-
- Davies J. C., Moskowitz S. M., Brown C., Horsley A., Mall M. A., McKone E. F., Plant B. J., Prais D., Ramsey B. W., Taylor-Cousar J. L.. et al. VX-659–Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018;379:1599–1611. doi: 10.1056/NEJMoa1807119. - DOI - PMC - PubMed
-
- Middleton P. G., Mall M. A., Dřevínek P., Lands L. C., McKone E. F., Polineni D., Ramsey B. W., Taylor-Cousar J. L., Tullis E., Vermeulen F.. et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N. Engl. J. Med. 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

