The Antinociceptive Effect of Nicorandil in Neuropathic and Nociceptive Pain is Partially Mediated via TRPV1/Opioidergic Signaling
- PMID: 40540176
- PMCID: PMC12433447
- DOI: 10.1007/s12035-025-05136-5
The Antinociceptive Effect of Nicorandil in Neuropathic and Nociceptive Pain is Partially Mediated via TRPV1/Opioidergic Signaling
Abstract
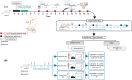
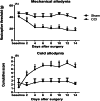
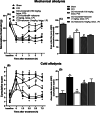
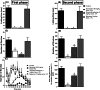
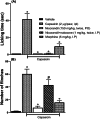
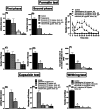
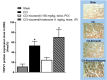
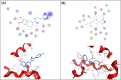
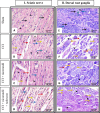
Neuropathic pain is a serious neurological disorder caused by lesioned somatosensory neurons characterized by multiple pathologies. Transient receptor potential vanilloid 1 (TRPV1) channels and opioid receptors are co-expressed in dorsal root ganglia (DRG) and play a crucial role in the development of neuropathic pain. Here, we investigated the possible involvement of TRPV1 channels and µ-opioid receptors in mediating the antinociception of the KATP opener, nicorandil, in neuropathic pain in four nociceptive models: chronic constriction injury of the sciatic nerve (CCI), formalin, capsaicin, and acetic acid writhing tests. Nicorandil (150 mg/kg, twice, 2 h apart, PO) administered to male rats (i) reversed the effects of CCI on nociceptive threshold and cumulative scores assessed by von Frey and acetone test, respectively; (ii) reduced licking time and number of flinches in biphasic formalin and capsaicin tests, and (iii) reduced the number of writhes in the acetic acid test; and (iv) combined nicorandil-capsaicin abolished acetic acid induced writhing response. Similarly, ipsilateral intraplantar injection of nicorandil (37.5 mg/paw, twice, ipl) inhibited nociceptive responses induced by capsaicin, formalin, and acetic acid. Immunohistochemical analysis revealed that nicorandil blunted the CCI-induced elevation of TRPV1 protein expression in DRG. The beneficial effects of nicorandil in all models were attenuated by naloxone. Molecular docking supported the interaction between nicorandil and TRPV1. Histologically, nicorandil improved the pathological changes induced by CCI in the sciatic nerve and DRG. Collectively, these results demonstrate that nicorandil exhibits antinociceptive effects in neuropathic and nociceptive pain via mechanisms involving TRPV1 modulation and opioid receptor signaling. Further investigation is warranted to explore the mechanism of action of nicorandil as an alternative treatment option for neuropathic pain.
Keywords: Neuropathic pain; Nicorandil; Opioidergic; TRPV1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

